Содержание
- 2. Corrosion is the gradual destruction of materials (usually metals) by chemical reaction with their environment. In
- 3. Corrosion can also occur in materials other than metals, such as ceramics or polymers, although in
- 4. Many structural alloys corrode merely from exposure to moisture in air, but the process can be
- 5. Metal corrosion is a spontaneous thermodynamic destruction (anodic oxidation) metal as a result of exposure to
- 6. In terms of redox reactions: nature of this interaction is reduced to the oxidation of the
- 7. Iron oxidation by atmospheric oxygen: 4Fe + 3O2 = 2Fe2O3 Corrosion of iron in aqueous solution
- 8. Steel Corrosion 1) Initial Oxidation Reaction: 2) Secondary Oxidation Reaction: rust
- 9. Classification of Corrosion By type of corrosive environment By the nature of destruction By the types
- 10. By operating conditions known the following types of electrochemical corrosion crevice corrosion contact corrosion corrosion fatigue
- 11. Crevice corrosion - electrochemical corrosion in cracks and gaps between the two metals, which enters the
- 12. Gas corrosion Atmospheric corrosion Liquid corrosion Soil corrosion Stray currents
- 13. Gas Corrosion - chemical corrosion of metals in gases at high temperatures (e.g., in the combustion
- 14. Continual Corrosion Uniform corrosion Uneven corrosion
- 15. Local corrosion By ulcers By points By spots
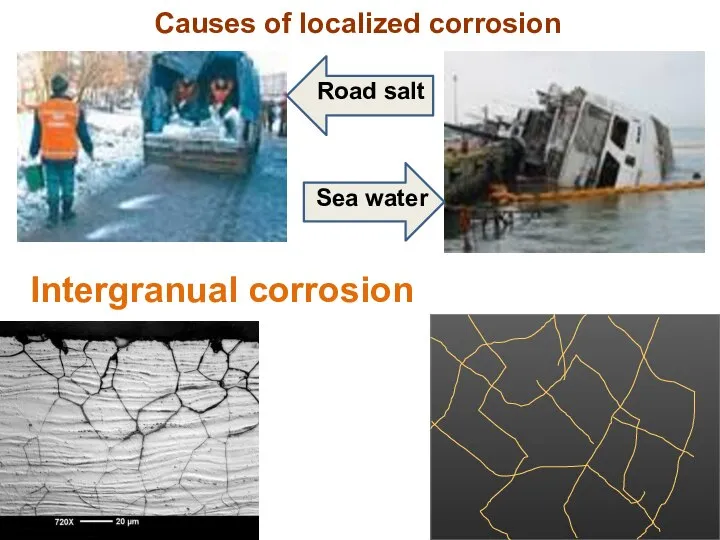
- 16. Causes of localized corrosion Intergranual corrosion Sea water Road salt
- 17. CHEMICAL CORROSION is the interaction of the metal with the environment in which the oxidation of
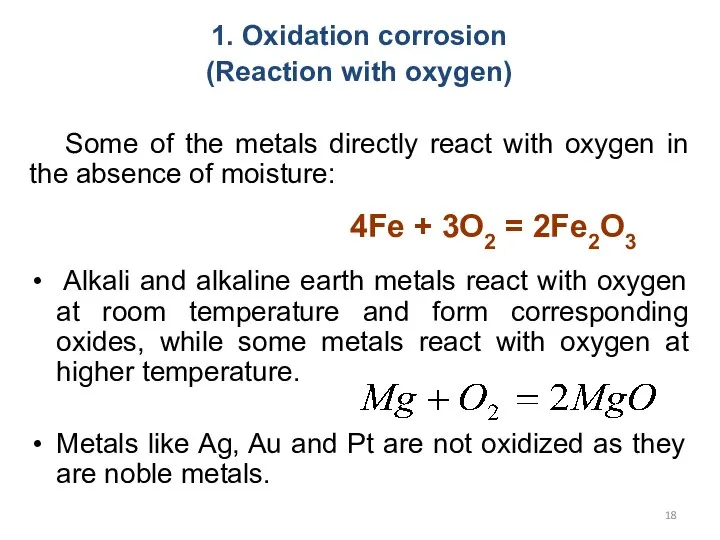
- 18. 1. Oxidation corrosion (Reaction with oxygen) Some of the metals directly react with oxygen in the
- 19. During oxidation of a metal, metal oxide is formed as a thin film on the metallic
- 20. Oxides of Pb, Al and Sn are stable and hence inhibit further corrosion. They form a
- 21. 2. Corrosion by other gases (Cl2, SO2, H2S, NOx) In dry atmosphere, these gases react with
- 22. 3. Liquid - metal corrosion In several industries, molten metal passes through metallic pipes and causes
- 23. ELECTROCHEMICAL CORROSION is anodic oxidation of the metal by environment elements in which the oxidation of
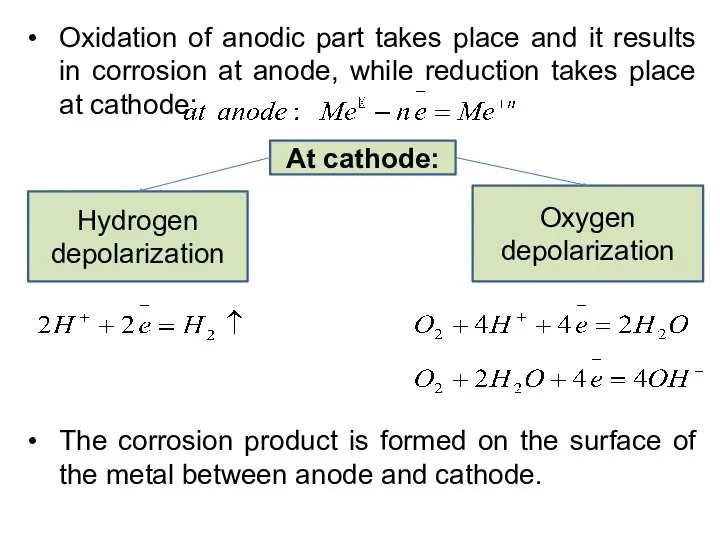
- 24. Oxidation of anodic part takes place and it results in corrosion at anode, while reduction takes
- 25. Mechanism: Electrochemical corrosion involves the flow of electron current between anodic and catholic regions. The anodic
- 26. Thus, electrochemical corrosion occurs: where a conducting liquid (water, acid, salt solutions) is in contact with
- 27. Oxygen reduction (acidic medium pH Fe + O2 + 4H+ = Fe2O3 * nH2O Oxygen reduction
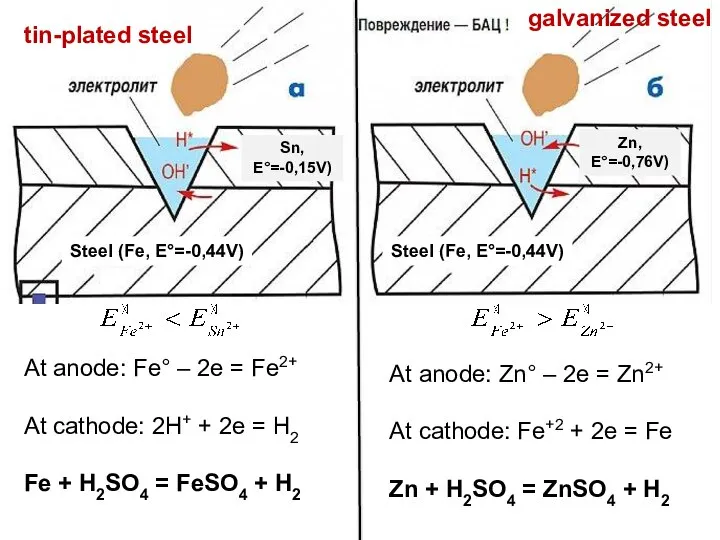
- 28. Steel (Fe, E°=-0,44V) Steel (Fe, E°=-0,44V) Zn, E°=-0,76V) Sn, E°=-0,15V) tin-plated steel At anode: Fe° –
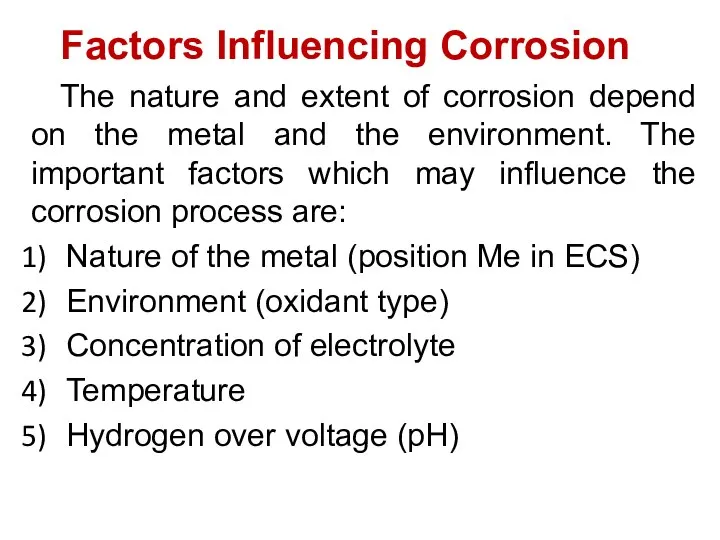
- 29. Factors Influencing Corrosion The nature and extent of corrosion depend on the metal and the environment.
- 30. 1. The position of metals in the electrochemical Series Lithium Potassium Calcium Sodium Magnesium Aluminum Zinc
- 31. potential area where any corrosion impossible, example Аu (1,5 В) area of corrosion with oxygen depolarization
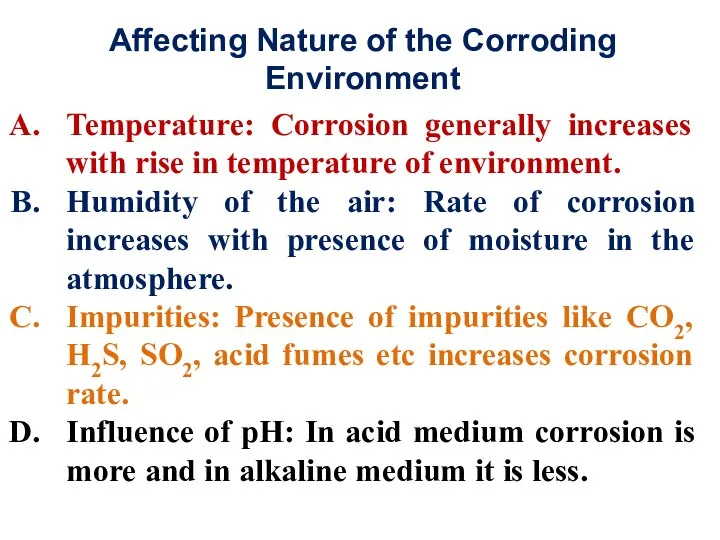
- 32. Affecting Nature of the Corroding Environment Temperature: Corrosion generally increases with rise in temperature of environment.
- 33. Group 1 - alkali metals - the lowest corrosion resistance 1 group of the sub-groups -
- 34. Group 3 - Aluminium - forming stable oxide film (but it is destroyed in solutions of
- 35. 5,6,7,8 group - by metals of subgroups have high ability to passivate and hence high resistance
- 36. PROTECTION METHODS Material selection Improvements in material Design of structures Alteration of environment Add inhibitors Galvanic
- 39. Скачать презентацию





























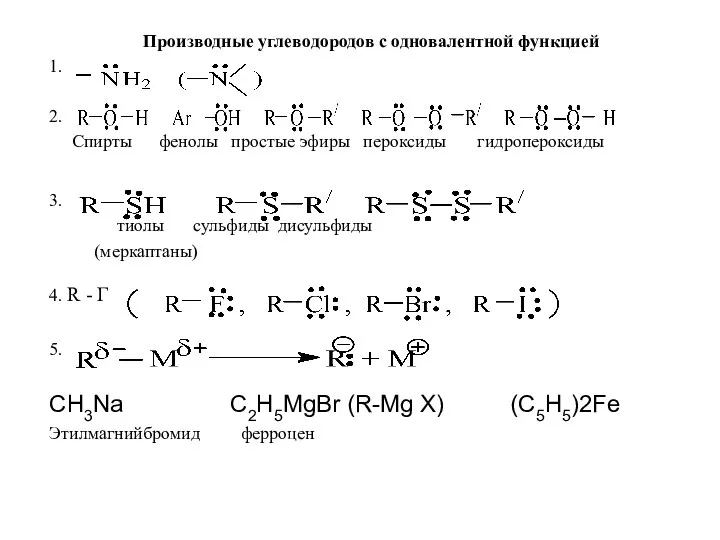 Производные углеводородов с одновалентной функцией
Производные углеводородов с одновалентной функцией Introduction to Periodic Table
Introduction to Periodic Table Нафта
Нафта  Физико-химические методы анализа
Физико-химические методы анализа Термохимия. Расчетные задачи. (Лекция 4.2)
Термохимия. Расчетные задачи. (Лекция 4.2) Старение полимеров. Процессы, протекающие при старении полимеров
Старение полимеров. Процессы, протекающие при старении полимеров Поверхностное натяжение. Поверхности раздела фаз
Поверхностное натяжение. Поверхности раздела фаз Твердые растворы Zn1,92-2хMg0,08Mn2xSiO4 и Zn1,76-2хMg0,24Mn2xSiO4: синтез и спектроскопические свойства
Твердые растворы Zn1,92-2хMg0,08Mn2xSiO4 и Zn1,76-2хMg0,24Mn2xSiO4: синтез и спектроскопические свойства Мұнайдың құрамындағы тұз мөлшерін анықтау
Мұнайдың құрамындағы тұз мөлшерін анықтау Синтетические лекарственные препараты
Синтетические лекарственные препараты Курс химии для основных академических направлений подготовки специалистов НИЯУ МИФИ
Курс химии для основных академических направлений подготовки специалистов НИЯУ МИФИ Рекомендации по подготовке учащихся к выполнению заданий различного уровня сложности ЕГЭ по химии
Рекомендации по подготовке учащихся к выполнению заданий различного уровня сложности ЕГЭ по химии СИБИРСКИЙ ФЕДЕРАЛЬНЫЙ УНИВЕРСИТЕТ Институт Нефти и Газа
СИБИРСКИЙ ФЕДЕРАЛЬНЫЙ УНИВЕРСИТЕТ Институт Нефти и Газа  Многоатомные спирты
Многоатомные спирты Презентация по Химии "Уран" - скачать смотреть
Презентация по Химии "Уран" - скачать смотреть  Горно-химическое сырье
Горно-химическое сырье Презентация по Химии "Волокнистые материалы вокруг нас" - скачать смотреть
Презентация по Химии "Волокнистые материалы вокруг нас" - скачать смотреть  Отношение масс элементов в веществе. Массовые доли элементов в веществе
Отношение масс элементов в веществе. Массовые доли элементов в веществе Химический состав клетки
Химический состав клетки Адам ағзасындағы химиялық элементтер
Адам ағзасындағы химиялық элементтер МОЛЕКУЛЯРНО - КИНЕТИЧЕСКАЯ ТЕОРИЯ идеального газа ОСНОВНЫЕ ПОЛОЖЕНИЯ МКТ. ИДЕАЛЬНЫЙ ГАЗ, ЕГО ПАРАМЕТРЫ. ОСНОВНОЕ УРАВНЕНИЕ МКТ.
МОЛЕКУЛЯРНО - КИНЕТИЧЕСКАЯ ТЕОРИЯ идеального газа ОСНОВНЫЕ ПОЛОЖЕНИЯ МКТ. ИДЕАЛЬНЫЙ ГАЗ, ЕГО ПАРАМЕТРЫ. ОСНОВНОЕ УРАВНЕНИЕ МКТ.  Органикалық қосылыстар
Органикалық қосылыстар Задачи Спирты. Альдегиды
Задачи Спирты. Альдегиды Калитина Тамара Михайловна учитель экологии, биологии МОУ СОШ №3 и учитель химии МОУ СОШ №2 с.Александров-Гай Саратовской области
Калитина Тамара Михайловна учитель экологии, биологии МОУ СОШ №3 и учитель химии МОУ СОШ №2 с.Александров-Гай Саратовской области  Автометаморфизм
Автометаморфизм Повышение эффективности разработки низкопродуктивных коллекторов самотлорского месторождения
Повышение эффективности разработки низкопродуктивных коллекторов самотлорского месторождения Диссоциация кислот, оснований и солей
Диссоциация кислот, оснований и солей Природные полимеры и продукты их химических превращений
Природные полимеры и продукты их химических превращений