Содержание
- 2. Methods of C–C – Bond Formation Reactions of nucleophiles with electrophiles Pericyclic reactions Radical reactions Other
- 3. Radical Reactions The best results if A = B Also possible if only one radical M.
- 4. Radical Reactions Giese Reaction N. P. Ramirez,J. C. Gonzalez-Gomez, Eur. J. Org. Chem. 2017, 2154–2163 I.
- 5. Radical Reactions Modification of Giese reaction T. Qin, L. R. Malins, J. T. Edwards, R. R.
- 7. Radical Reactions Programmed radical cross-coupling (RCC)! Is there an alternative for innate radical cross couplings (RCC)?
- 8. Radical Reactions Key steps of RCC J. M. Smith, S. J. Harwood, P. S. Baran, Acc.
- 9. Primary, secondary and tertiary acids can be used Usual as well as α, β – unsaturated
- 11. Decarboxylative Alkenylations (Another possibility) H. Chen, S. Sun, X. Liao, Org. Lett. 2019, 21, 3625−3630
- 12. Decarboxylative (Hetero)Arylations F. Sandfort, M. J. O’Neill, J.Cornella, L. Wimmer, P. S. Baran, Angew. Chem. Int.
- 14. Another Possibility of (Hetero)Arylations Particiularly interesting for the synthesis of fluorinated compounds Synthesis of the sulfones
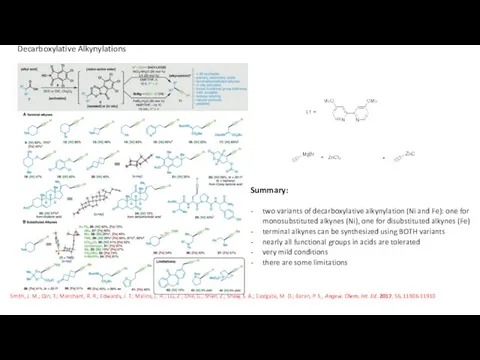
- 15. two variants of decarboxylative alkynylation (Ni and Fe): one for monosubstituted alkynes (Ni), one for disubstituted
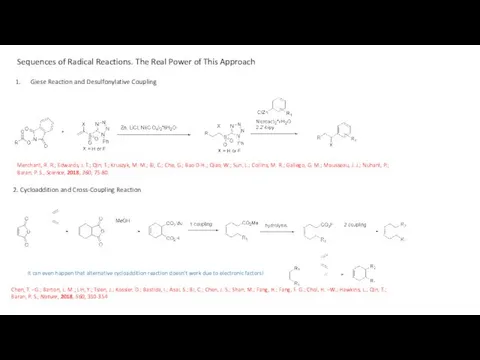
- 16. Sequences of Radical Reactions. The Real Power of This Approach Giese Reaction and Desulfonylative Coupling Merchant,
- 17. Synthesis of Amino Acids Ni, S.; Garrido-Castro, A. F.; Merchant, R. R.; deGruyter, J. N.; Schmitt,
- 19. Скачать презентацию














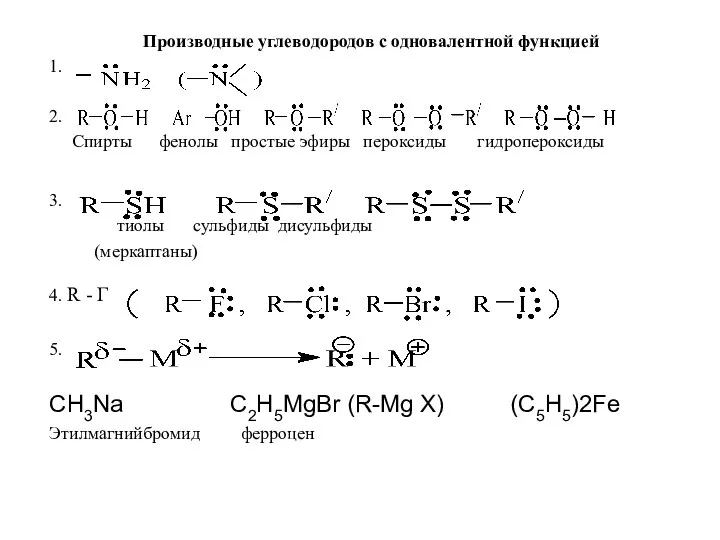 Производные углеводородов с одновалентной функцией
Производные углеводородов с одновалентной функцией Introduction to Periodic Table
Introduction to Periodic Table Нафта
Нафта  Физико-химические методы анализа
Физико-химические методы анализа Термохимия. Расчетные задачи. (Лекция 4.2)
Термохимия. Расчетные задачи. (Лекция 4.2) Старение полимеров. Процессы, протекающие при старении полимеров
Старение полимеров. Процессы, протекающие при старении полимеров Поверхностное натяжение. Поверхности раздела фаз
Поверхностное натяжение. Поверхности раздела фаз Твердые растворы Zn1,92-2хMg0,08Mn2xSiO4 и Zn1,76-2хMg0,24Mn2xSiO4: синтез и спектроскопические свойства
Твердые растворы Zn1,92-2хMg0,08Mn2xSiO4 и Zn1,76-2хMg0,24Mn2xSiO4: синтез и спектроскопические свойства Мұнайдың құрамындағы тұз мөлшерін анықтау
Мұнайдың құрамындағы тұз мөлшерін анықтау Синтетические лекарственные препараты
Синтетические лекарственные препараты Курс химии для основных академических направлений подготовки специалистов НИЯУ МИФИ
Курс химии для основных академических направлений подготовки специалистов НИЯУ МИФИ Рекомендации по подготовке учащихся к выполнению заданий различного уровня сложности ЕГЭ по химии
Рекомендации по подготовке учащихся к выполнению заданий различного уровня сложности ЕГЭ по химии СИБИРСКИЙ ФЕДЕРАЛЬНЫЙ УНИВЕРСИТЕТ Институт Нефти и Газа
СИБИРСКИЙ ФЕДЕРАЛЬНЫЙ УНИВЕРСИТЕТ Институт Нефти и Газа  Многоатомные спирты
Многоатомные спирты Презентация по Химии "Уран" - скачать смотреть
Презентация по Химии "Уран" - скачать смотреть  Горно-химическое сырье
Горно-химическое сырье Презентация по Химии "Волокнистые материалы вокруг нас" - скачать смотреть
Презентация по Химии "Волокнистые материалы вокруг нас" - скачать смотреть  Отношение масс элементов в веществе. Массовые доли элементов в веществе
Отношение масс элементов в веществе. Массовые доли элементов в веществе Химический состав клетки
Химический состав клетки Адам ағзасындағы химиялық элементтер
Адам ағзасындағы химиялық элементтер МОЛЕКУЛЯРНО - КИНЕТИЧЕСКАЯ ТЕОРИЯ идеального газа ОСНОВНЫЕ ПОЛОЖЕНИЯ МКТ. ИДЕАЛЬНЫЙ ГАЗ, ЕГО ПАРАМЕТРЫ. ОСНОВНОЕ УРАВНЕНИЕ МКТ.
МОЛЕКУЛЯРНО - КИНЕТИЧЕСКАЯ ТЕОРИЯ идеального газа ОСНОВНЫЕ ПОЛОЖЕНИЯ МКТ. ИДЕАЛЬНЫЙ ГАЗ, ЕГО ПАРАМЕТРЫ. ОСНОВНОЕ УРАВНЕНИЕ МКТ.  Органикалық қосылыстар
Органикалық қосылыстар Задачи Спирты. Альдегиды
Задачи Спирты. Альдегиды Калитина Тамара Михайловна учитель экологии, биологии МОУ СОШ №3 и учитель химии МОУ СОШ №2 с.Александров-Гай Саратовской области
Калитина Тамара Михайловна учитель экологии, биологии МОУ СОШ №3 и учитель химии МОУ СОШ №2 с.Александров-Гай Саратовской области  Автометаморфизм
Автометаморфизм Повышение эффективности разработки низкопродуктивных коллекторов самотлорского месторождения
Повышение эффективности разработки низкопродуктивных коллекторов самотлорского месторождения Диссоциация кислот, оснований и солей
Диссоциация кислот, оснований и солей Природные полимеры и продукты их химических превращений
Природные полимеры и продукты их химических превращений