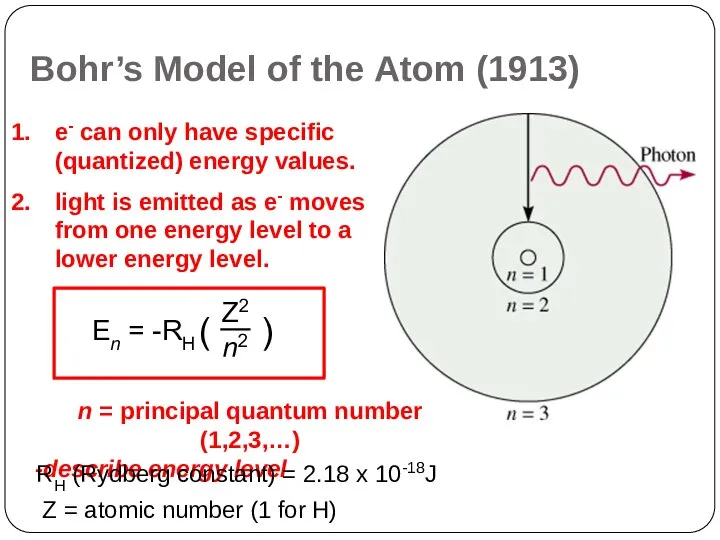
Bohr’s Model of the Atom (1913)
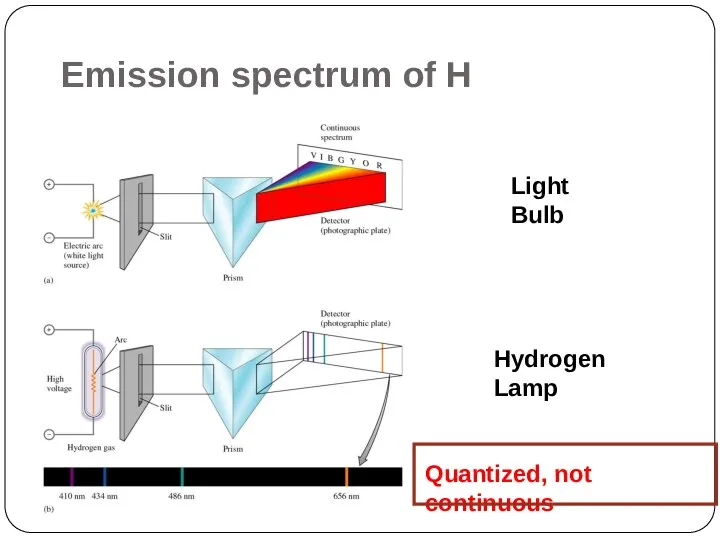
Electrons cannot have just any amount
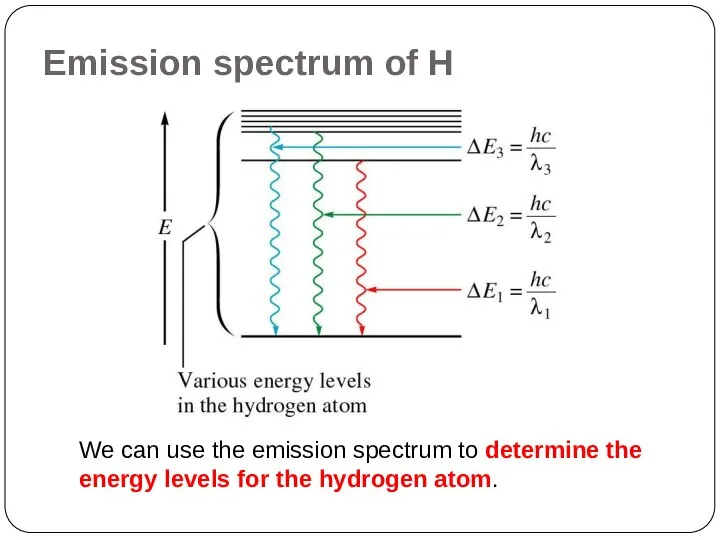
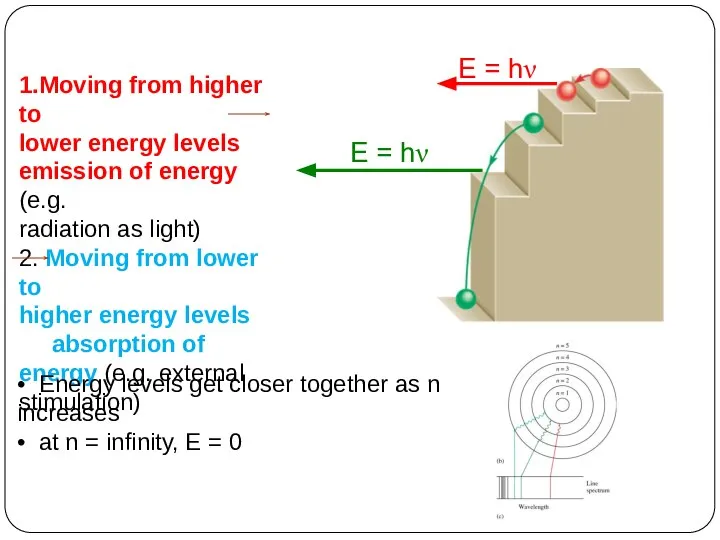
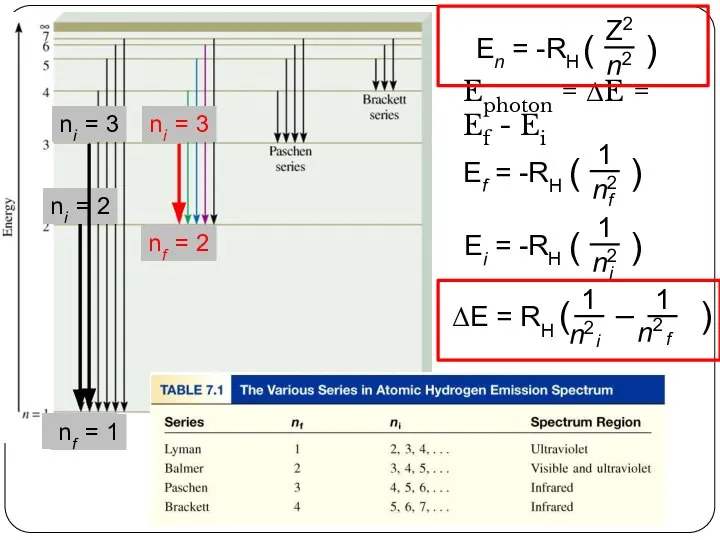
of energy but can have only certain specified amount; i.e. the energy of an electron is quantized. The specified energy values for an atom are called its energy levels.
As an electron moves instantaneously from one energy level to another, there are no intermediate stages.
Niels Henrik David Bohr was a Danish physicist who made foundational contributions to understanding atomic structure and quantum mechanics, for which he received the Nobel Prize in Physics in 1922.
http://www.youtube.com/watch?v=Ic8OnvRonb0






 Тропосфера та її вплив на поширення радіохвиль
Тропосфера та її вплив на поширення радіохвиль Андрианова Е.Ю.учитель физики. Москва ГБОУ СОШ «Школа здоровья «№404. Виды теплопередачи.
Андрианова Е.Ю.учитель физики. Москва ГБОУ СОШ «Школа здоровья «№404. Виды теплопередачи.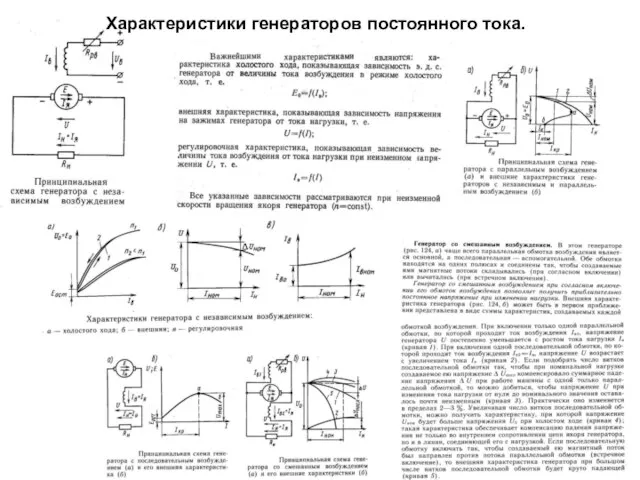 Характеристики генераторов постоянного тока. Судовое электроосвещение. (Билет 25)
Характеристики генераторов постоянного тока. Судовое электроосвещение. (Билет 25) Газовые законы
Газовые законы Энергия заряженного конденсатора. Применение конденсаторов
Энергия заряженного конденсатора. Применение конденсаторов Эксперимент и моделирование – основные физические методы исследовАния природы
Эксперимент и моделирование – основные физические методы исследовАния природы Service Training, VK-21
Service Training, VK-21 Плавание тел. Водоизмещение судов
Плавание тел. Водоизмещение судов Динамика вращательного движения
Динамика вращательного движения Дисперсиялык талдаудын бір факторлы параметрлік емес үқсастығы критерий - Крускал Уоллис критерийі
Дисперсиялык талдаудын бір факторлы параметрлік емес үқсастығы критерий - Крускал Уоллис критерийі Закон Архимеда
Закон Архимеда  Тепловое излучение и люминесценция
Тепловое излучение и люминесценция Physics and chemistry of surface phenomena
Physics and chemistry of surface phenomena Виштовхувальна сила в рідинах і газах. Закон Архімеда
Виштовхувальна сила в рідинах і газах. Закон Архімеда Магнитное поле и его характеристики
Магнитное поле и его характеристики Первый закон термодинамики. Газовые смеси
Первый закон термодинамики. Газовые смеси Современные попытки создания вечного двигателя
Современные попытки создания вечного двигателя Червячные передачи
Червячные передачи Переходные процессы в энергетических системах (ЭЭС)
Переходные процессы в энергетических системах (ЭЭС) Что является источником энергии? Что является источником энергии?
Что является источником энергии? Что является источником энергии? Виды и назначение токарных резцов (7 класс)
Виды и назначение токарных резцов (7 класс) Институт физики Казанского федерального университета
Институт физики Казанского федерального университета Световые явления в живой и неживой природе
Световые явления в живой и неживой природе Приводы агрегатов авиационного двигателя. (Тема 9)
Приводы агрегатов авиационного двигателя. (Тема 9) Активные слои тонкопленочных приборов на основе аморфного кремния
Активные слои тонкопленочных приборов на основе аморфного кремния Конструкции распределительных устройств. (Лекция 15)
Конструкции распределительных устройств. (Лекция 15) Презентация по физике На тему: Термоядерная реакция
Презентация по физике На тему: Термоядерная реакция Момент силы
Момент силы