Содержание
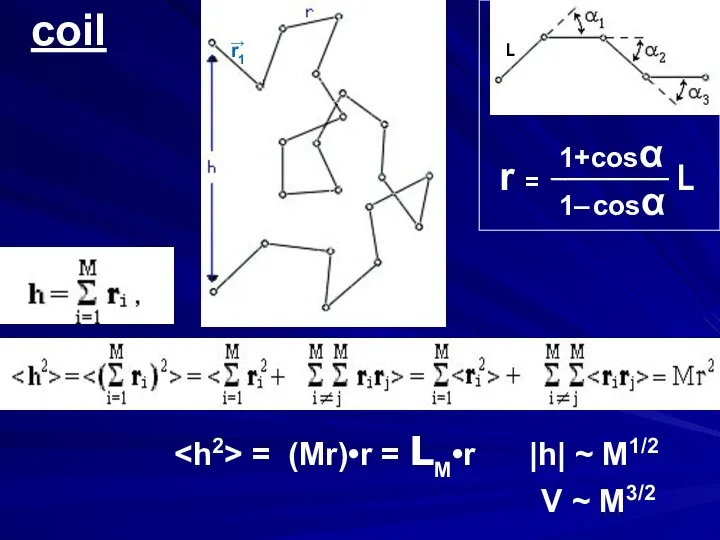
- 2. coil r = ________ L 1+cosα 1– cosα = (Mr)•r = LM•r |h| ~ M1/2 V
- 3. Linus Carl Pauling (1901-94) — Nobel Prizes: 1954, 62 Werner Kuhn (1899 - 1963) Robert Brainard
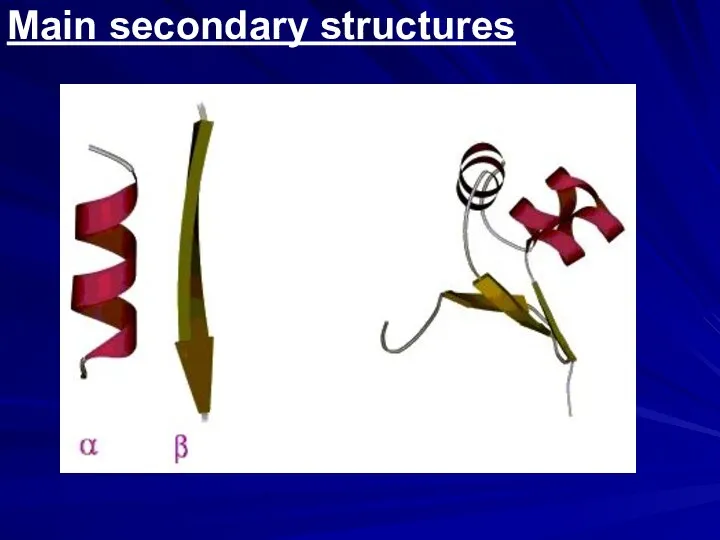
- 4. Main secondary structures
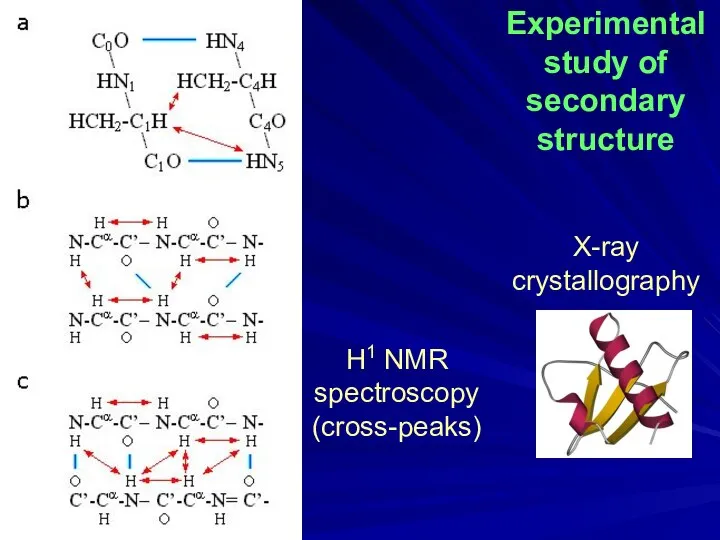
- 5. H1 NMR spectroscopy (cross-peaks) Experimental study of secondary structure X-ray crystallography
- 6. Far UV CD spectra (peptide groups) IR spectra (“amid I”, C=O bond) Experimental study of secondary
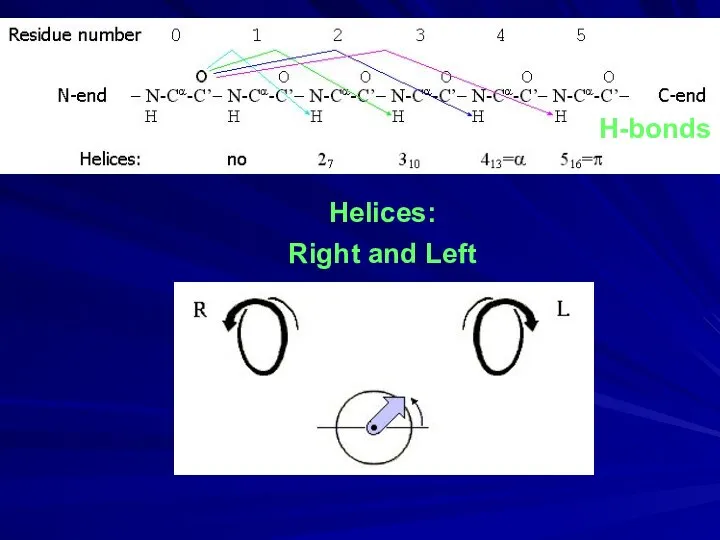
- 7. Helices: Right and Left H-bonds
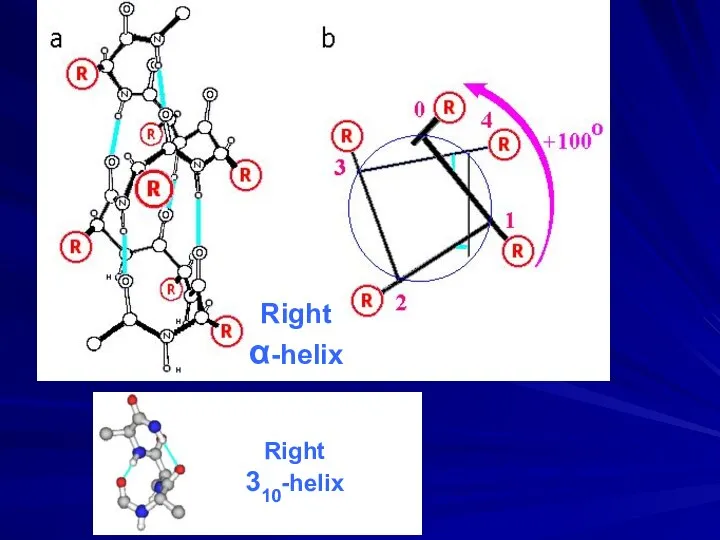
- 8. Right α-helix Right 310-helix
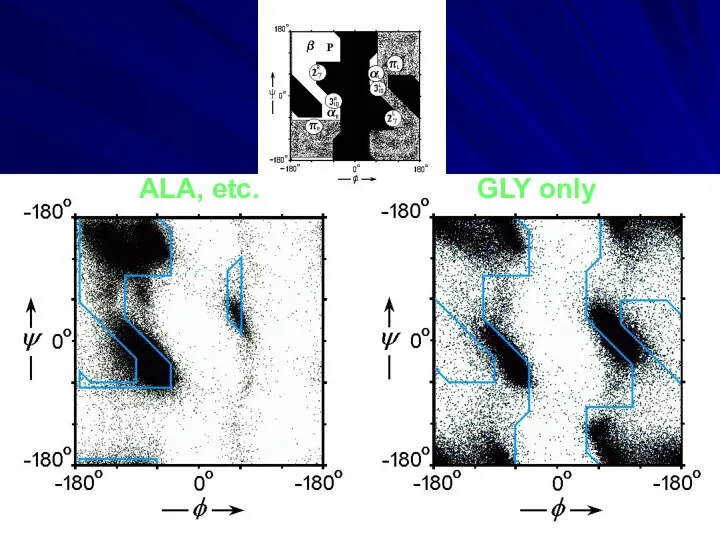
- 10. ALA, etc. GLY only
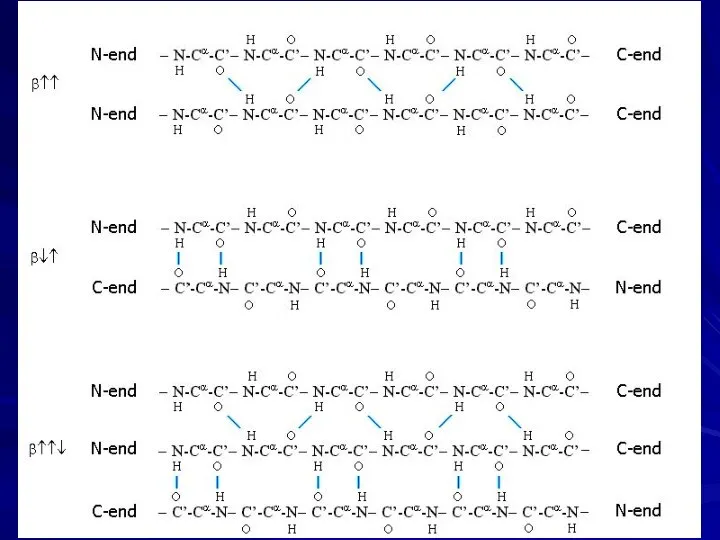
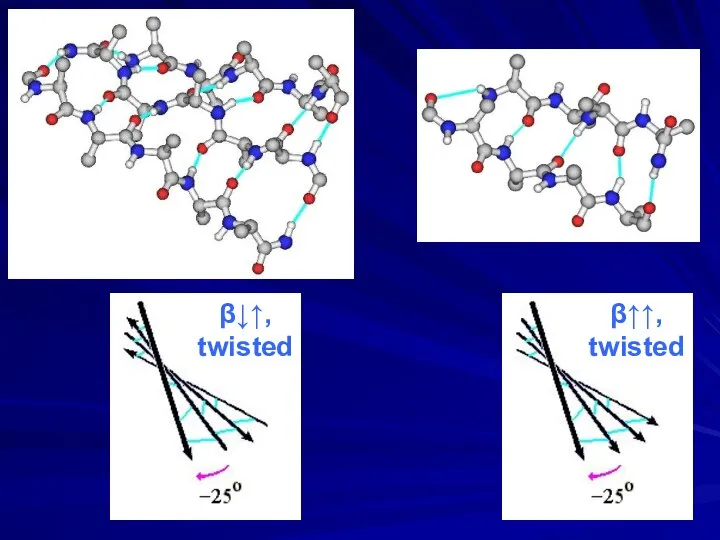
- 12. β↑↑, twisted β↓↑, twisted
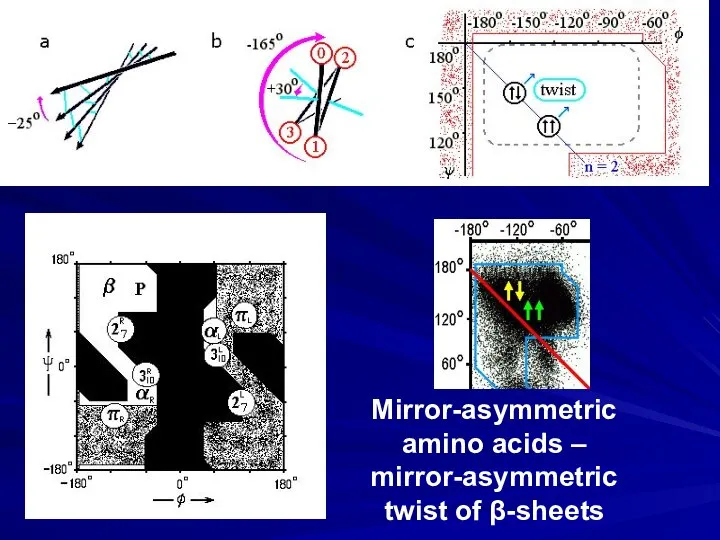
- 13. Mirror-asymmetric amino acids – mirror-asymmetric twist of β-sheets
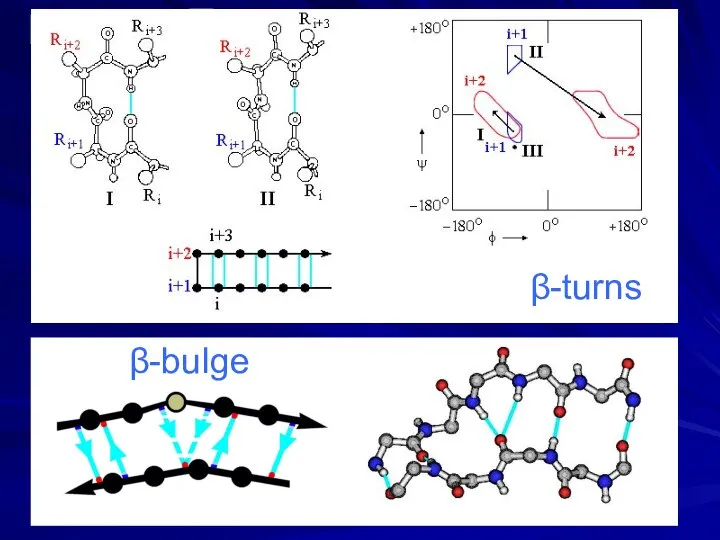
- 14. β-turns β-bulge
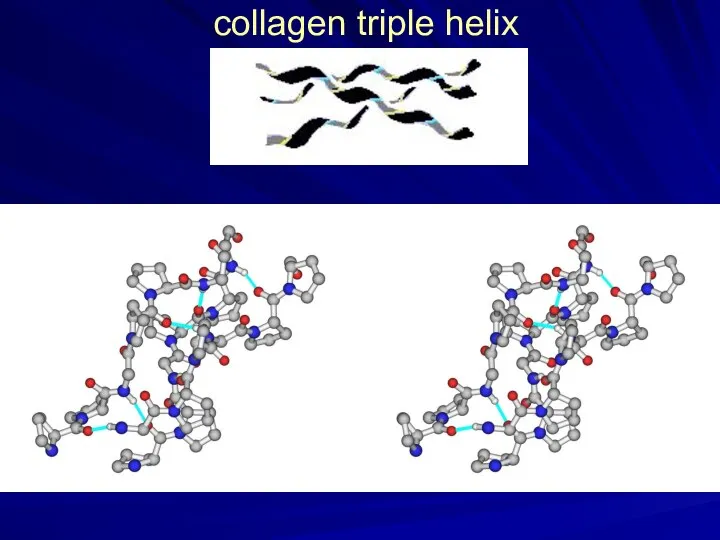
- 15. collagen triple helix
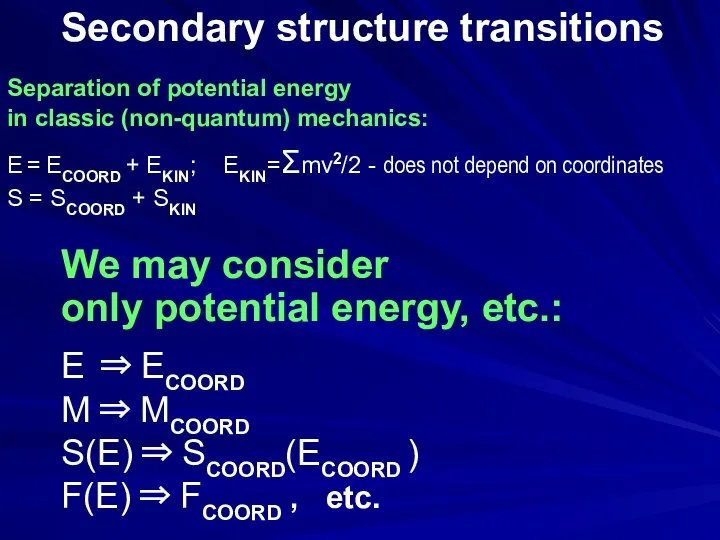
- 16. Secondary structure transitions We may consider only potential energy, etc.: E ⇒ ECOORD M ⇒ MCOORD
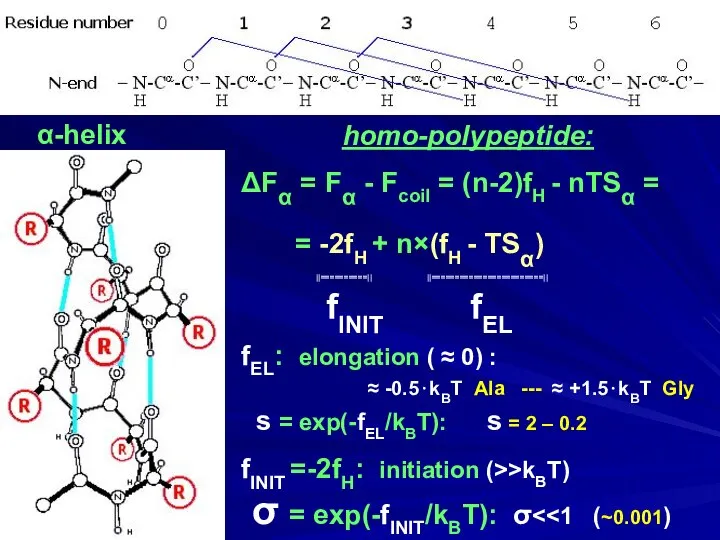
- 17. α-helix homo-polypeptide: ΔFα = Fα - Fcoil = (n-2)fH - nTSα = = -2fH + n×(fH
- 18. α-helix homo-polypeptide: ΔFα = Fα - Fcoil = (n-2)fH - nTSα = = -2fH + n×(fH
- 19. Average lengths n0 of helix and coil regions at mid-transition (when fEL=0, fINIT>>kBT): N n Eα
- 20. Width of helix-coil transition When fEL changes: IF n0 ×fEL IF n0 ×fEL >> +kBT; i.e.,
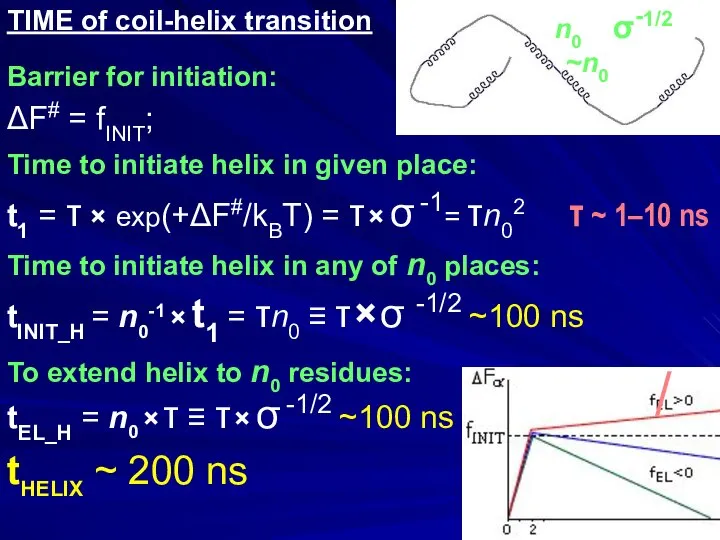
- 21. TIME of coil-helix transition Barrier for initiation: ΔF# = fINIT; Time to initiate helix in given
- 22. TIME of coil – stable β-hairpin transition Barrier for initiation: ΔF# = fTURN ≈ fINIT_α; Time
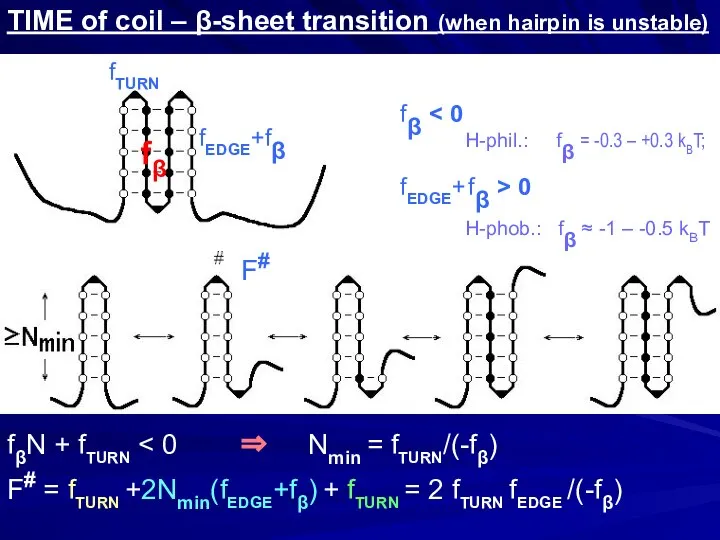
- 23. TIME of coil – β-sheet transition (when hairpin is unstable) fβ fTURN fEDGE+fβ fβN + fTURN
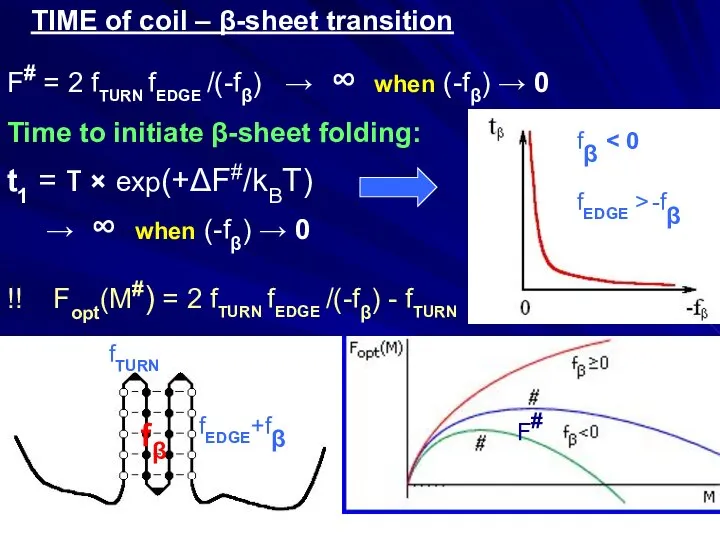
- 24. TIME of coil – β-sheet transition fβ fTURN fEDGE+fβ F# F# = 2 fTURN fEDGE /(-fβ)
- 25. The End
- 27. Скачать презентацию








 Изотопная геология (введение)
Изотопная геология (введение) Диэлектрические потери
Диэлектрические потери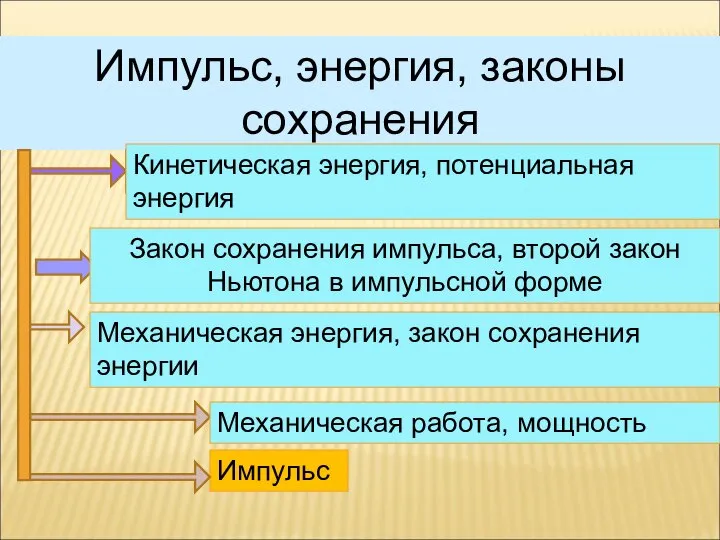 Импульс, энергия, законы сохранения. Решение задач
Импульс, энергия, законы сохранения. Решение задач Классификация методов синтеза наноматериалов
Классификация методов синтеза наноматериалов Презентація з фізичного практикуму на тему:”Фізика й науково-технічний прогрес” підготувала: учениця 11-А класу Харківської ЗОШ І-ІІІ ст №102 Антіпова Марія
Презентація з фізичного практикуму на тему:”Фізика й науково-технічний прогрес” підготувала: учениця 11-А класу Харківської ЗОШ І-ІІІ ст №102 Антіпова Марія  Презентация по физике "Напряжение" - скачать
Презентация по физике "Напряжение" - скачать  Зубчатые передачи
Зубчатые передачи Нагревание проводников электрическим током. Закон Джоуля-Ленца
Нагревание проводников электрическим током. Закон Джоуля-Ленца Штормгласс - предсказатель бурь
Штормгласс - предсказатель бурь Передача и использование электроэнергии
Передача и использование электроэнергии Электрическое поле. (лекция 1а)
Электрическое поле. (лекция 1а) Механическое движение и его виды
Механическое движение и его виды Действие магнитного поля на проводник с током. Электрический двигатель
Действие магнитного поля на проводник с током. Электрический двигатель Физические основы термодинамики
Физические основы термодинамики Теплопроводность при наличии внутренних источников теплоты
Теплопроводность при наличии внутренних источников теплоты Ультразвук и инфразвук в природе
Ультразвук и инфразвук в природе Вес воздуха. Атмосферное давление. Выполнил: студент 4 курса Специальность физика Д.Е. Таргоний
Вес воздуха. Атмосферное давление. Выполнил: студент 4 курса Специальность физика Д.Е. Таргоний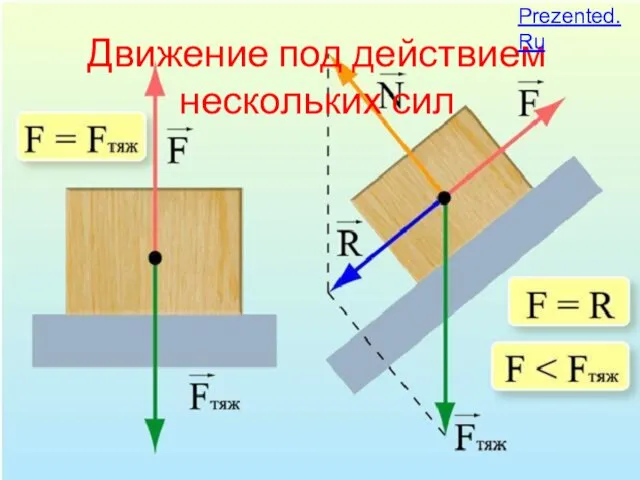 Движение под действием нескольких сил
Движение под действием нескольких сил Современные проблемы технической физики. Самые дорогие научные проекты
Современные проблемы технической физики. Самые дорогие научные проекты Макс Карл Эрнст Людвиг Планк (23.05.1858-04.10.1947)
Макс Карл Эрнст Людвиг Планк (23.05.1858-04.10.1947) Лабораторные работы по физике 8 класс
Лабораторные работы по физике 8 класс Принципы радиосвязи. (8 класс)
Принципы радиосвязи. (8 класс) Индикаторлық галоидты жанарғы
Индикаторлық галоидты жанарғы Электрическое поле
Электрическое поле ПРОЕКТ «Определение средней скорости моего движения.» Выполнил ученик 7Б класса
ПРОЕКТ «Определение средней скорости моего движения.» Выполнил ученик 7Б класса  Светодиодные лампы
Светодиодные лампы Внутренняя энергия. Термодинамика
Внутренняя энергия. Термодинамика Термодинамика поверхностных явлений. (Часть 2)
Термодинамика поверхностных явлений. (Часть 2)