Содержание
- 2. Basic information The stratospheric ozone layer began to form soon after the onset of oxygen producing
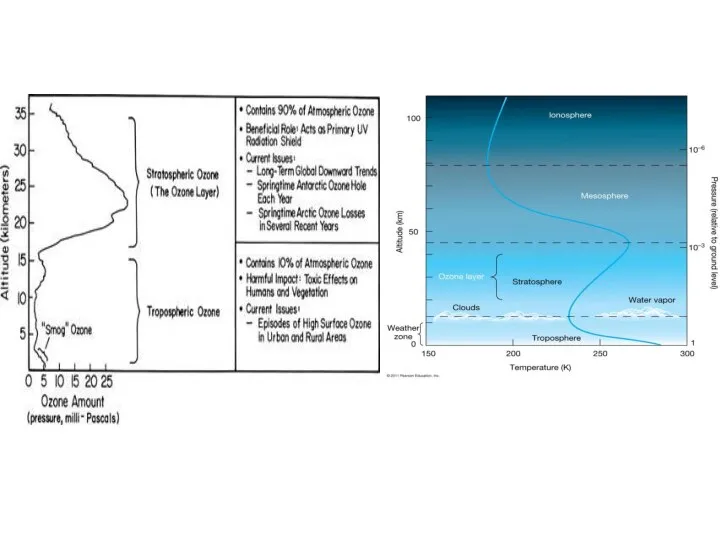
- 4. Basic information A measure of the quantity of ozone in the air is the ozone column
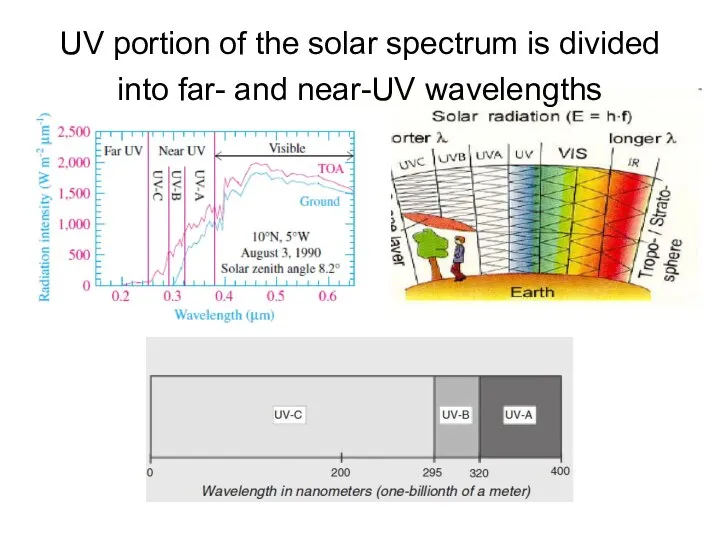
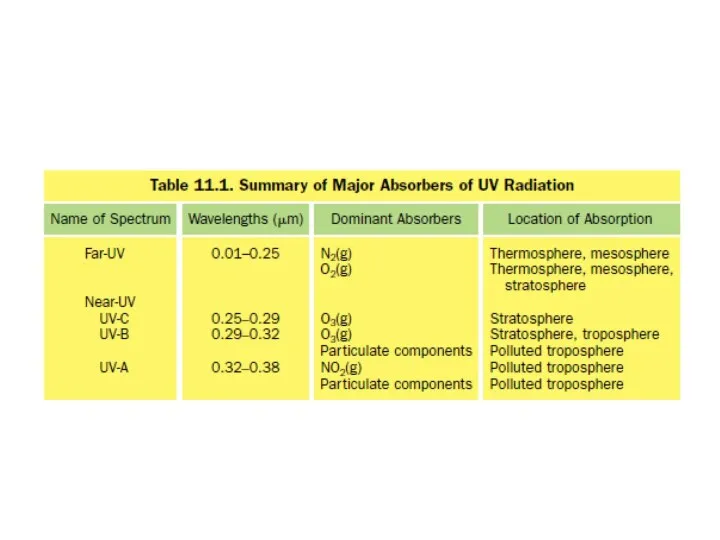
- 5. UV portion of the solar spectrum is divided into far- and near-UV wavelengths
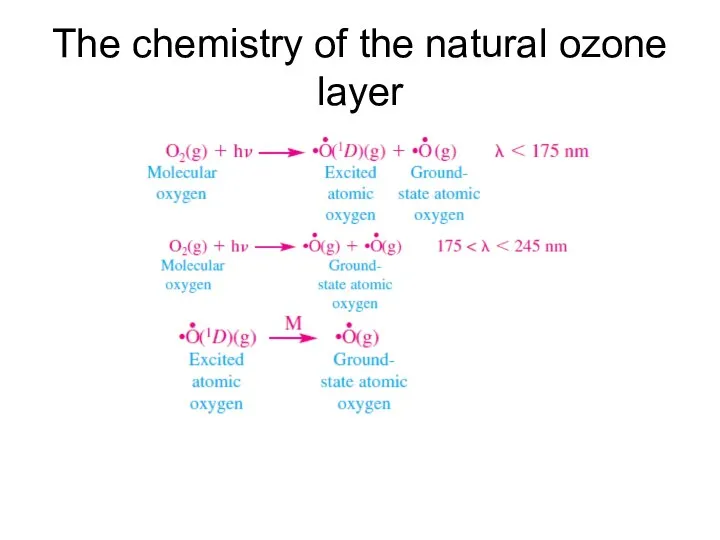
- 7. The chemistry of the natural ozone layer
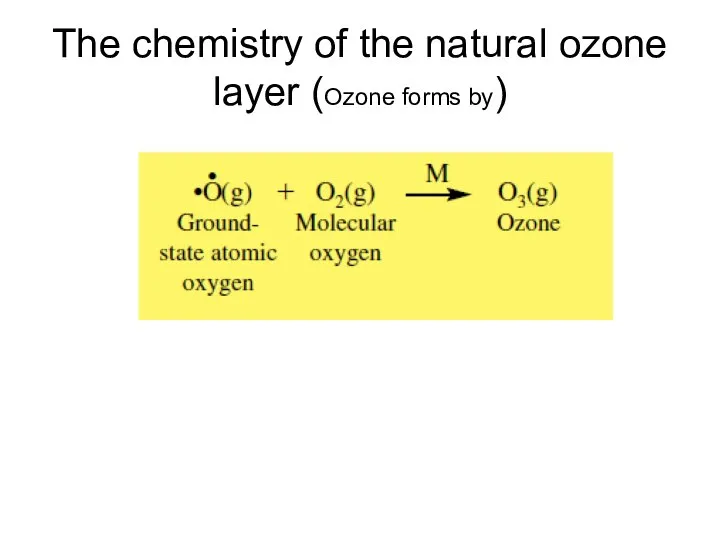
- 8. The chemistry of the natural ozone layer (Ozone forms by)
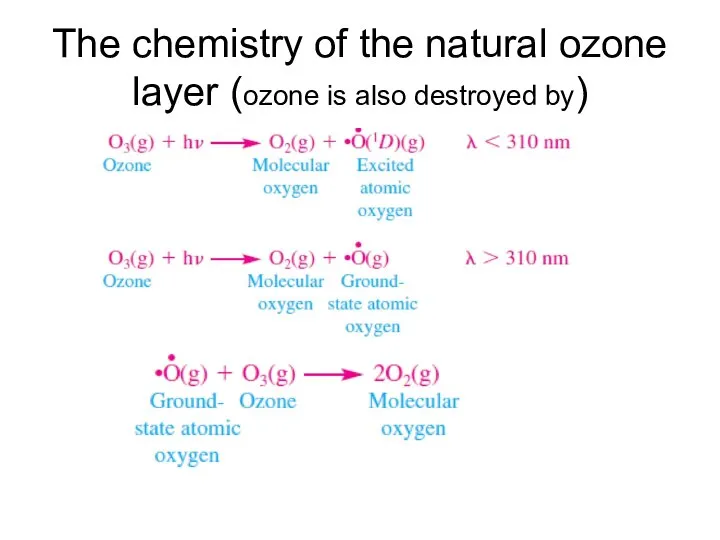
- 9. The chemistry of the natural ozone layer (ozone is also destroyed by)
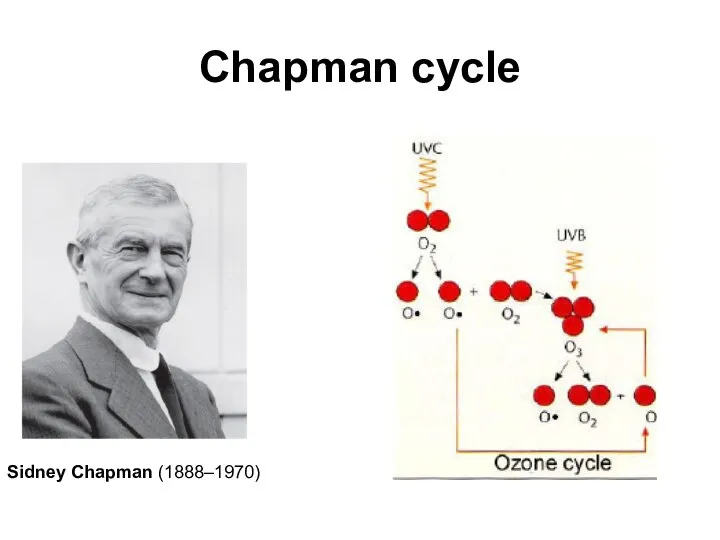
- 10. Chapman cycle Sidney Chapman (1888–1970)
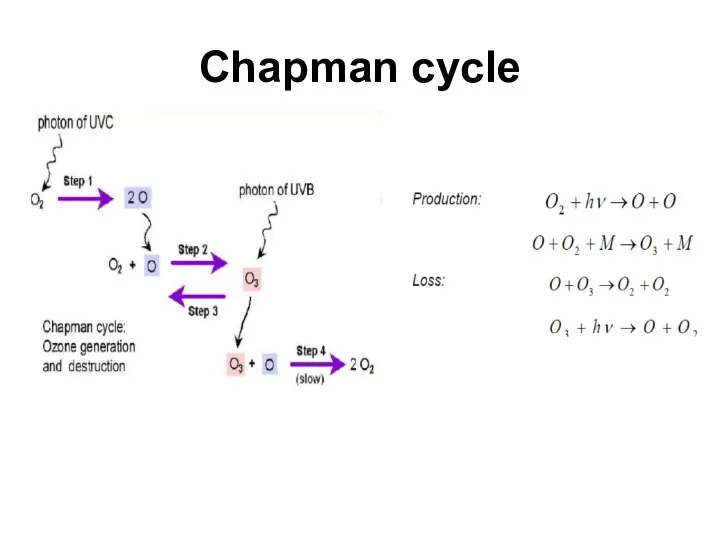
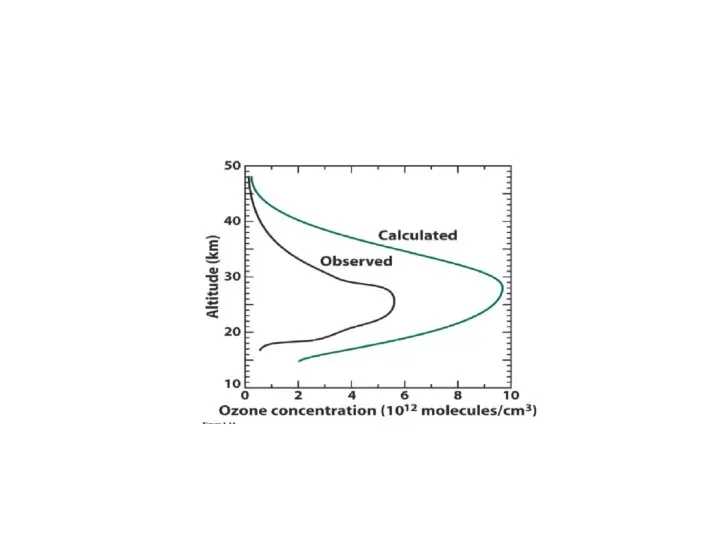
- 11. Chapman cycle
- 13. Effects of Nitrogen on the Natural Ozone Layer Oxides of nitrogen [NO(g) and NO2(g)] naturally destroy
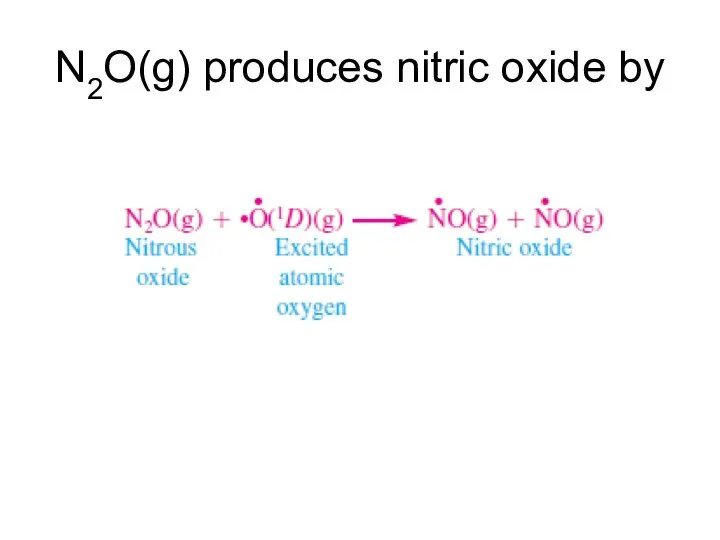
- 14. N2O(g) produces nitric oxide by
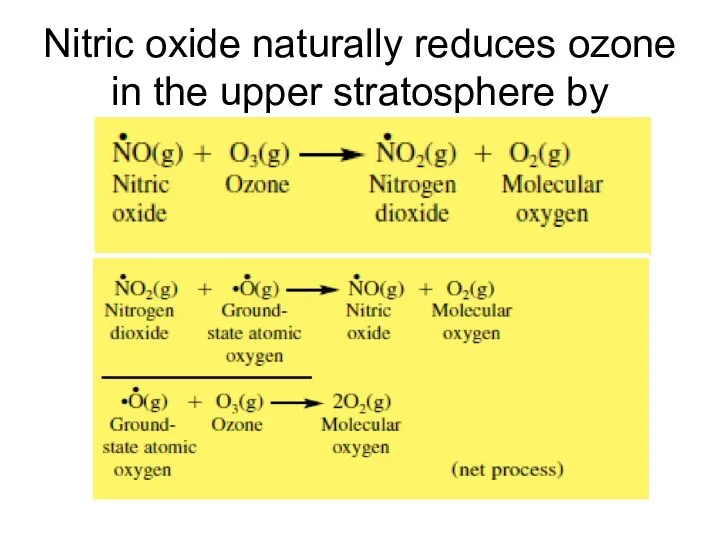
- 15. Nitric oxide naturally reduces ozone in the upper stratosphere by
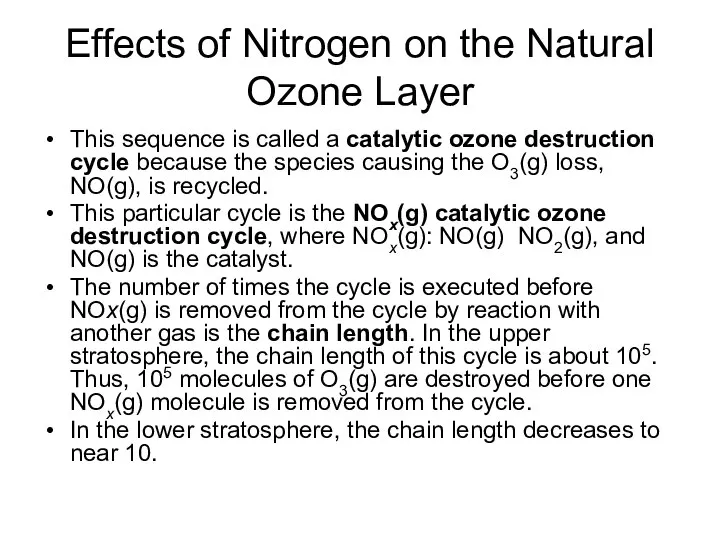
- 16. Effects of Nitrogen on the Natural Ozone Layer This sequence is called a catalytic ozone destruction
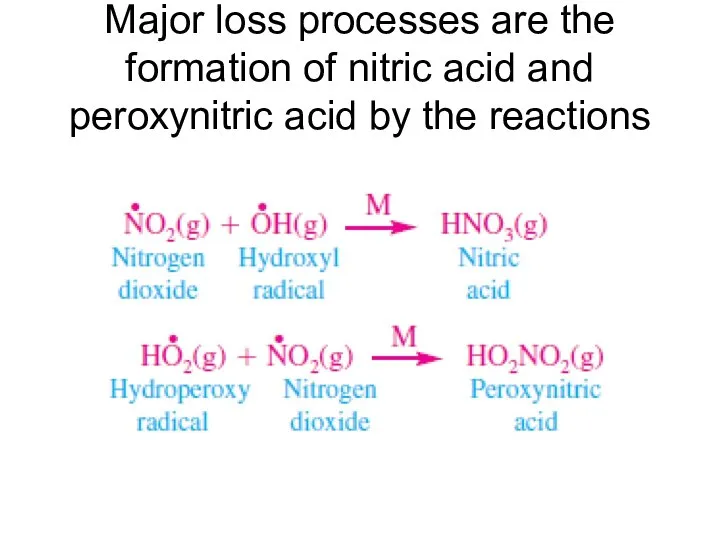
- 17. Major loss processes are the formation of nitric acid and peroxynitric acid by the reactions
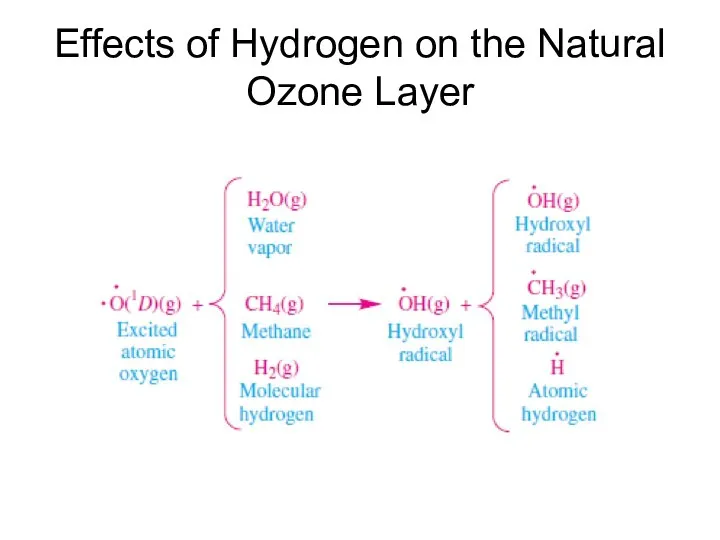
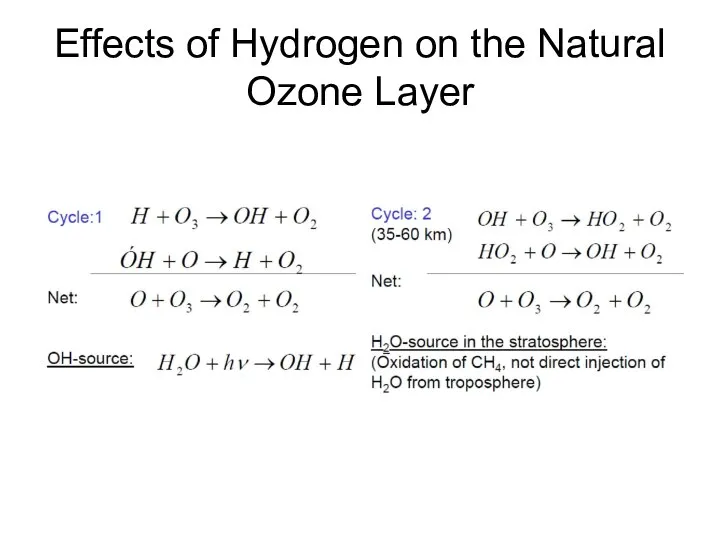
- 18. Effects of Hydrogen on the Natural Ozone Layer
- 19. Effects of Hydrogen on the Natural Ozone Layer The hydroxyl radical participates in an HOx(g) catalytic
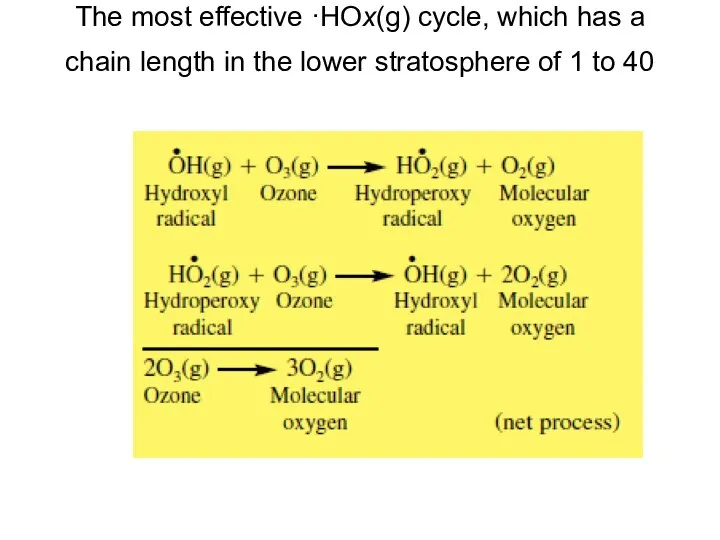
- 20. The most effective ·HOx(g) cycle, which has a chain length in the lower stratosphere of 1
- 21. Effects of Hydrogen on the Natural Ozone Layer
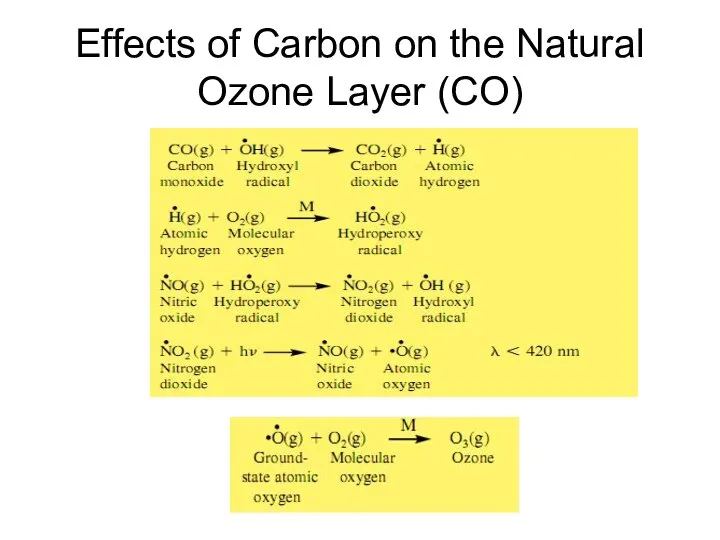
- 22. Effects of Carbon on the Natural Ozone Layer (CO)
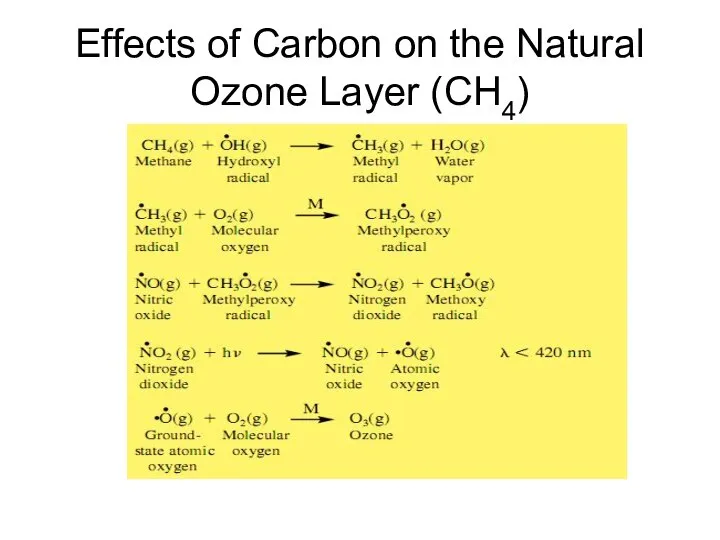
- 23. Effects of Carbon on the Natural Ozone Layer (CH4)
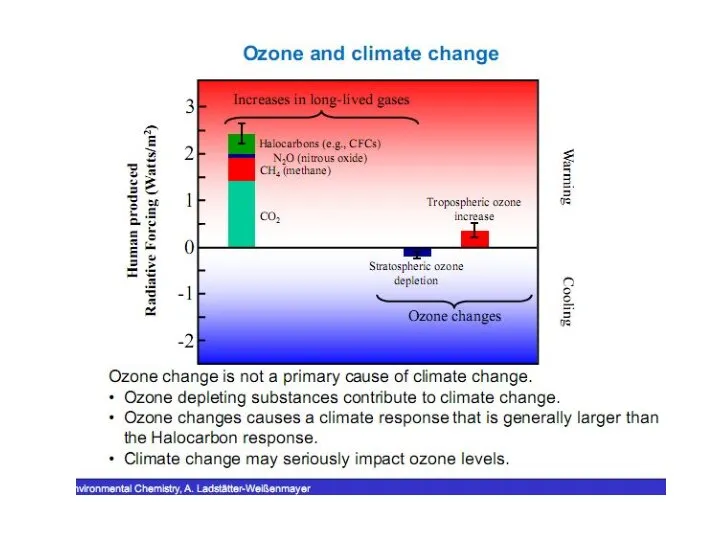
- 24. Changes on a Global Scale Between 1979 and 2000, the global stratospheric ozone column abundance decreased
- 25. CFCs and Related Compounds The compounds that play the most important role in reducing stratospheric ozone
- 26. CFCs and Related Compounds These compounds are non-flammable, tasteless and odourless, and chemically stable. Their other
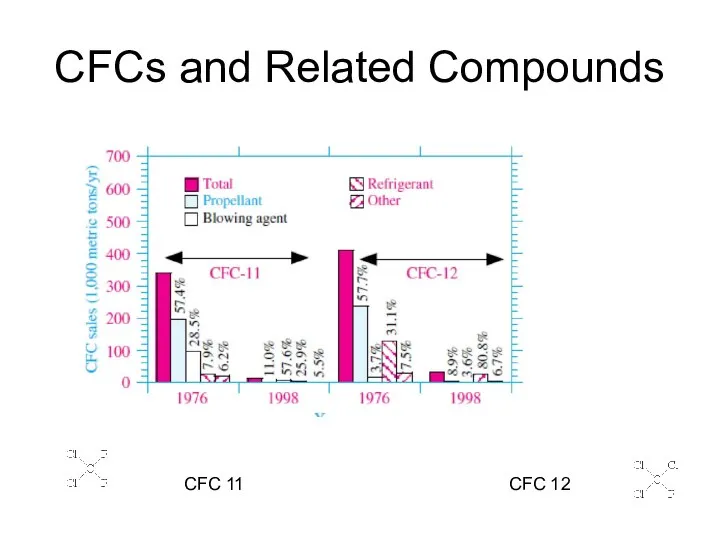
- 28. CFCs and Related Compounds CFC 11 CFC 12
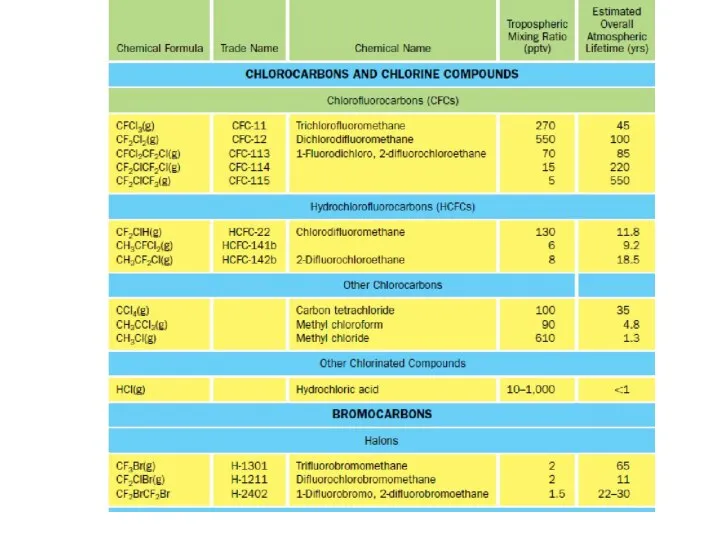
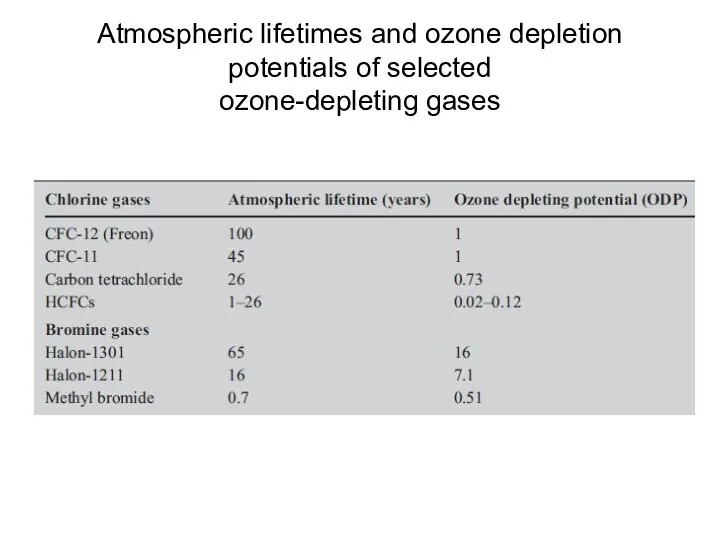
- 29. Atmospheric lifetimes and ozone depletion potentials of selected ozone-depleting gases
- 30. Other Chlorine Compounds Hydrochlorofluorocarbons (HCFCs) are another subset of chlorocarbons. The hydrogen atom allows HCFCs to
- 31. Bromine Compounds The primary source of stratospheric bromine is methyl bromide [CH3Br(g)], which is produced biogenically
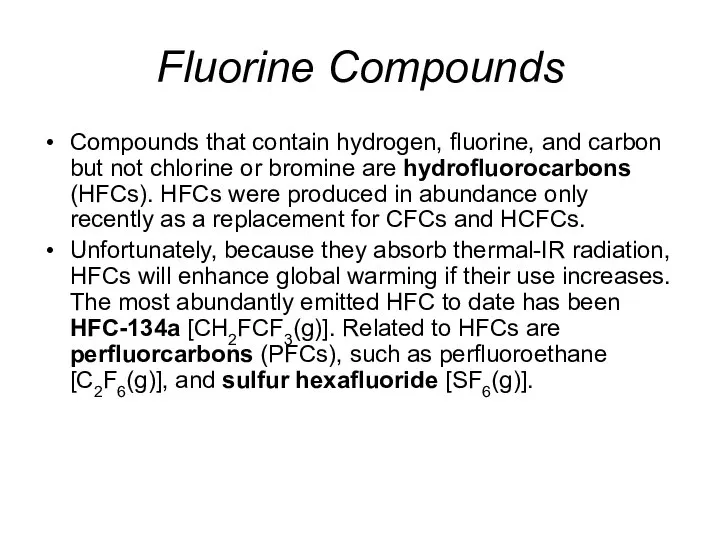
- 32. Fluorine Compounds Compounds that contain hydrogen, fluorine, and carbon but not chlorine or bromine are hydrofluorocarbons
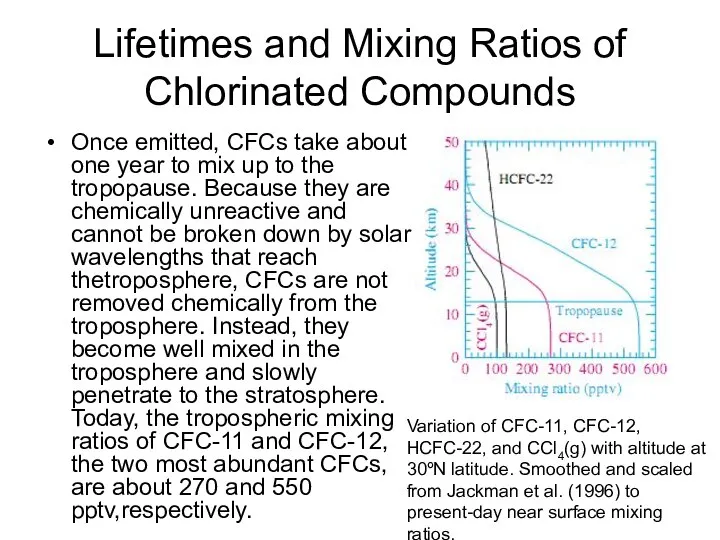
- 33. Lifetimes and Mixing Ratios of Chlorinated Compounds Once emitted, CFCs take about one year to mix
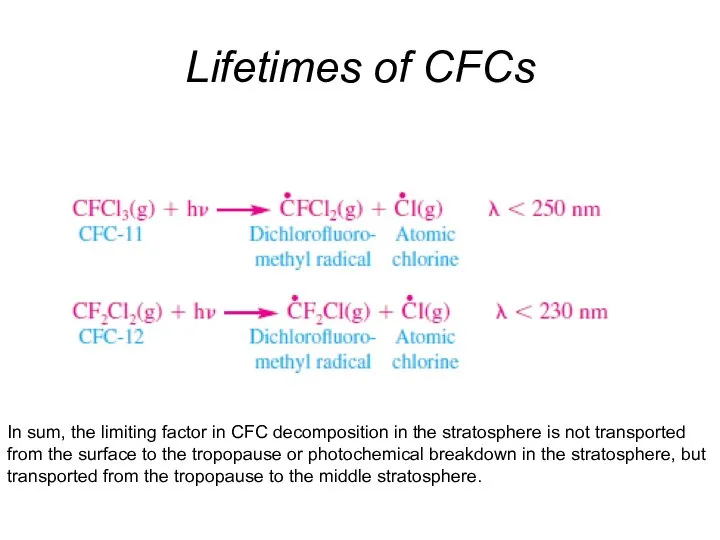
- 34. Lifetimes of CFCs Because the stratosphere is one large temperature inversion, vertical transport of ozone through
- 35. Lifetimes of CFCs In sum, the limiting factor in CFC decomposition in the stratosphere is not
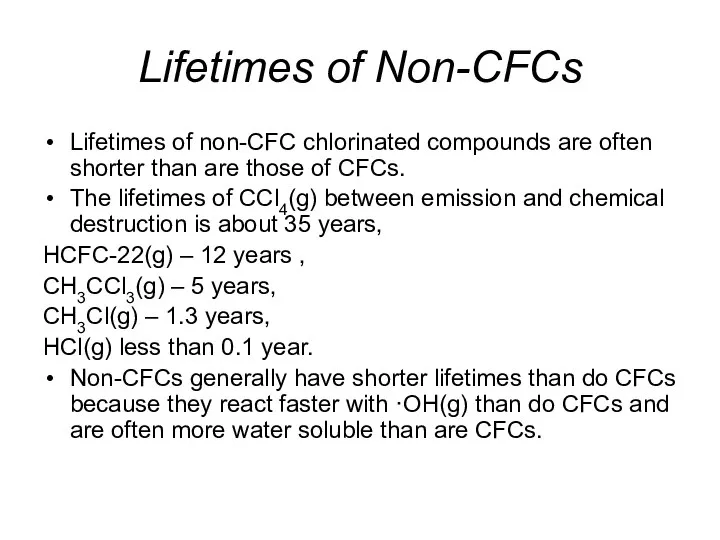
- 36. Lifetimes of Non-CFCs Lifetimes of non-CFC chlorinated compounds are often shorter than are those of CFCs.
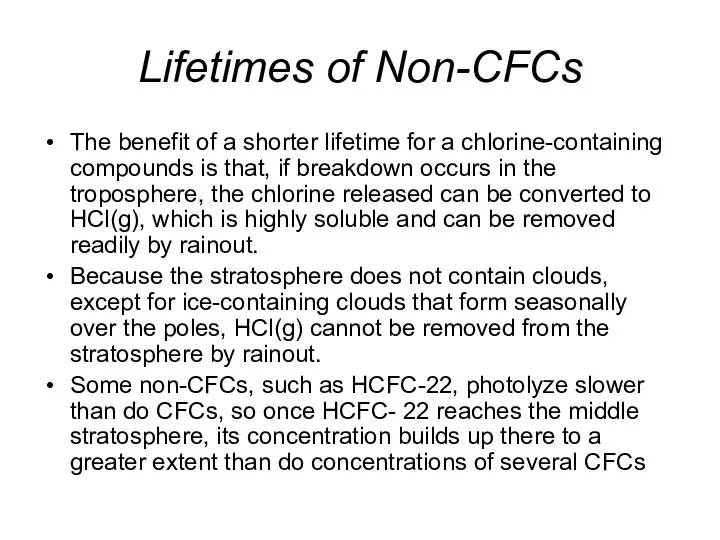
- 37. Lifetimes of Non-CFCs The benefit of a shorter lifetime for a chlorine-containing compounds is that, if
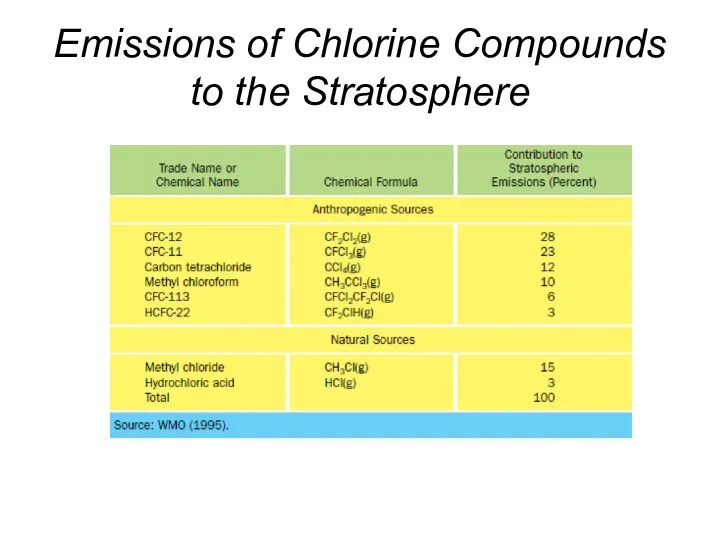
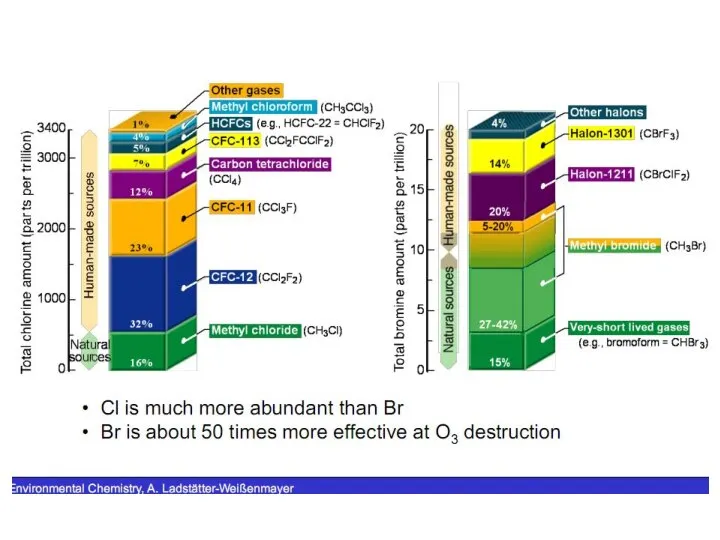
- 38. Emissions of Chlorine Compounds to the Stratosphere
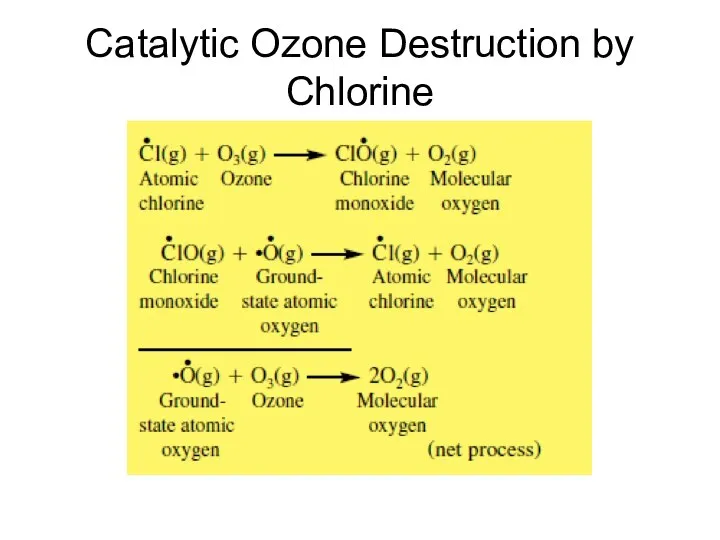
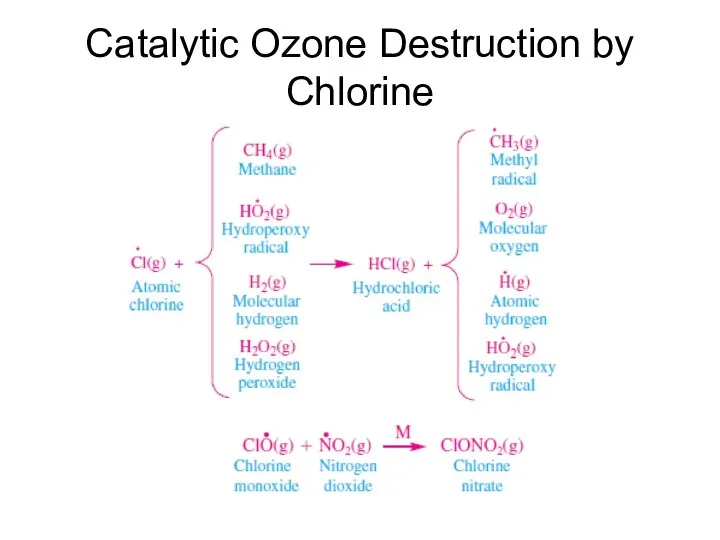
- 39. Catalytic Ozone Destruction by Chlorine
- 40. Catalytic Ozone Destruction by Chlorine
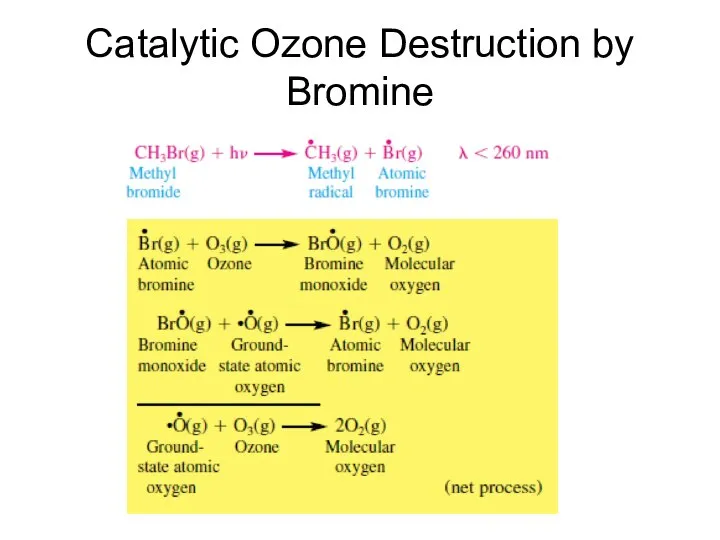
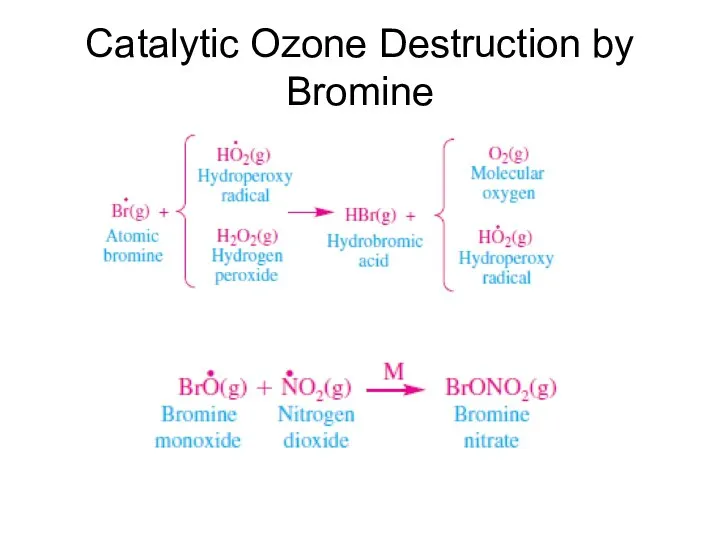
- 41. Catalytic Ozone Destruction by Bromine
- 42. Catalytic Ozone Destruction by Bromine
- 44. Effects on Humans Increases in UV-B radiation have potential to affect the skin, eyes, immune system
- 45. Effects on Skin The severity of effects of UV-B radiation on skin depends on skin pigmentation.
- 46. Effects on Eyes With respect to the eye, the cornea, which covers the iris and the
- 47. Effects on the Immune System Enhanced UV-B radiation has been linked to suppression of these cells,
- 48. Effects on the Global Carbon and Nitrogen Cycles Changes in UV-B radiation affect the global carbon
- 49. Effects on Tropospheric Ozone Increases in UV-B radiation increase photolysis rates of UV-B absorbing gases, such
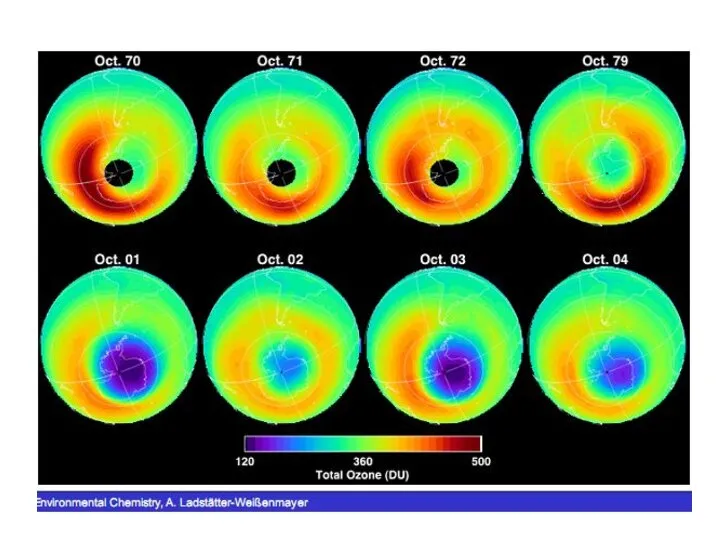
- 52. Arctic stratospheric ozone A great deal of scientific effort has gone into understanding the physical and
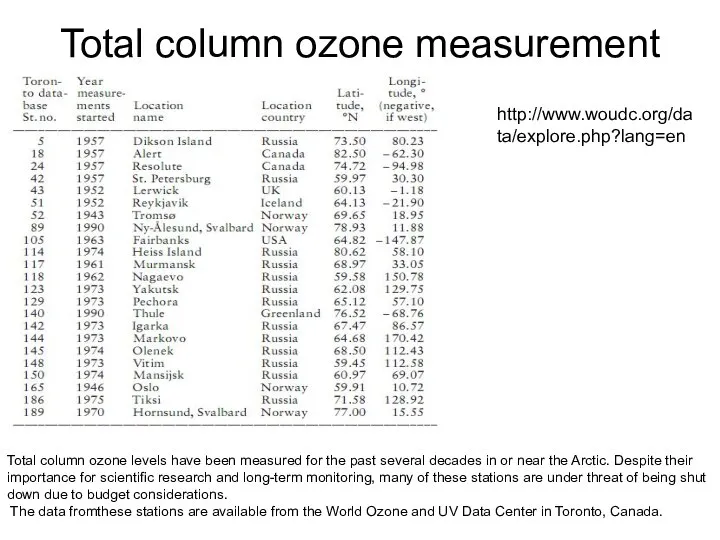
- 53. Total column ozone measurement stations Total column ozone levels have been measured for the past several
- 55. Скачать презентацию








 Презентация на тему Как нам жить вместе с природой? Урок по окружающему миру 4 класс
Презентация на тему Как нам жить вместе с природой? Урок по окружающему миру 4 класс  Свет как экологический фактор
Свет как экологический фактор Определение видового состава древесно-кустарниковых растений, используемых в озеленении территории школы
Определение видового состава древесно-кустарниковых растений, используемых в озеленении территории школы Презентация Кто такие рыбы?
Презентация Кто такие рыбы? Ландшафтное предложение по благоустройству берёзовой рощи
Ландшафтное предложение по благоустройству берёзовой рощи Характеристика современного состояния биосферы
Характеристика современного состояния биосферы Охрана природы и охраняемые территории
Охрана природы и охраняемые территории Загрязнение природной среды
Загрязнение природной среды Шенталинское школьное лесничество
Шенталинское школьное лесничество Аттестационная работа. Особо охраняемые территории Ставропольского края. Формирование экологической культуры у учащихся
Аттестационная работа. Особо охраняемые территории Ставропольского края. Формирование экологической культуры у учащихся Редкие животные, занесенные в красную книгу
Редкие животные, занесенные в красную книгу Среды жизни на Земле и экологические факторы. 9 класс
Среды жизни на Земле и экологические факторы. 9 класс Презентация Праздники народов России
Презентация Праздники народов России 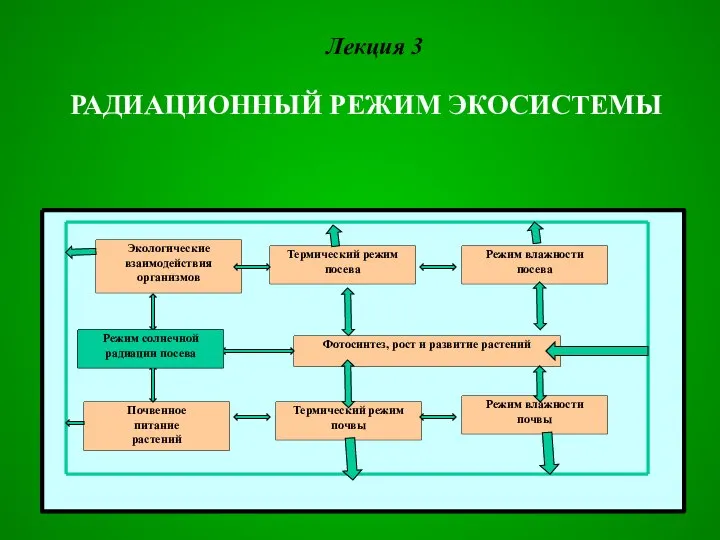 Радиационный режим экосистемы
Радиационный режим экосистемы Современное экологическое состояние Угличского водохранилища: проблемы и пути решения
Современное экологическое состояние Угличского водохранилища: проблемы и пути решения Принципы интеграции аквакультуры и экологического туризма
Принципы интеграции аквакультуры и экологического туризма Законодательное регулирование видов пользования лесом. Заготовка древесины
Законодательное регулирование видов пользования лесом. Заготовка древесины Государственный природный биосферный заповедник "Даурский"
Государственный природный биосферный заповедник "Даурский" Красная книга. Охрана редких видов Ленинградской области
Красная книга. Охрана редких видов Ленинградской области Устойчивость и динамика экосистем
Устойчивость и динамика экосистем Санитарно-гигиеническое значение атмосферного воздуха
Санитарно-гигиеническое значение атмосферного воздуха Адміністративно-територіальний устрій Ніжинського району
Адміністративно-територіальний устрій Ніжинського району Заказники Новосибирской области
Заказники Новосибирской области VI Геоэкологическая школа Галдым
VI Геоэкологическая школа Галдым Экосистема и биогеоценоз
Экосистема и биогеоценоз Мурманская региональная организация «За безопасность окружающей среды»
Мурманская региональная организация «За безопасность окружающей среды» Persistent organic pollutants
Persistent organic pollutants Энергетика мен экологияның өзара әрекеттесу проблемалары
Энергетика мен экологияның өзара әрекеттесу проблемалары