Содержание
- 2. 1 Pfizer BioNTech FDA Approval Letter Vaccine BNT162b - Comirnaty https://www.fda.gov/media/151710/download
- 3. 1 Pfizer BioNTech FDA Approval Letter Vaccine BNT162b - Comirnaty https://www.fda.gov/media/151710/download
- 4. 1 Pfizer BioNTech FDA Approval Letter Vaccine BNT162b - Comirnaty https://www.fda.gov/media/151710/download
- 5. 1 Pfizer BioNTech FDA Approval Letter Vaccine BNT162b - Comirnaty https://www.fda.gov/media/151710/download
- 6. 1 Pfizer BioNTech FDA Approval Letter Vaccine BNT162b - COMIRNATY https://www.fda.gov/media/151710/download
- 7. 1 Pfizer BioNTech FDA Approval Letter Vaccine BNT162b - COMIRNATY https://www.fda.gov/media/151710/download
- 8. 1 Pfizer BioNTech FDA Approval Letter Vaccine BNT162b - Comirnaty https://www.fda.gov/media/151710/download
- 9. July 7. 2020 – Moderna’s Patent for Current Vaccine Modified Polynucleotide or Production of Secreted Proteins
- 10. MODERNA US 10,703,789, B2. July 7, 2020 Articles 219, 220: LNP may be a gel, like
- 11. E MODERNA US 10,703,789, B2. July 7, 2020 MODERNA US 10,703,789, B2. July 7, 2020
- 12. https://www.nature.com/articles/s41428-020-0350-9
- 13. https://www.nature.com/articles/s41428-020-0350-9 HUNDREDS of Studies of Graphene-Oxide Incorporated Hydrogels
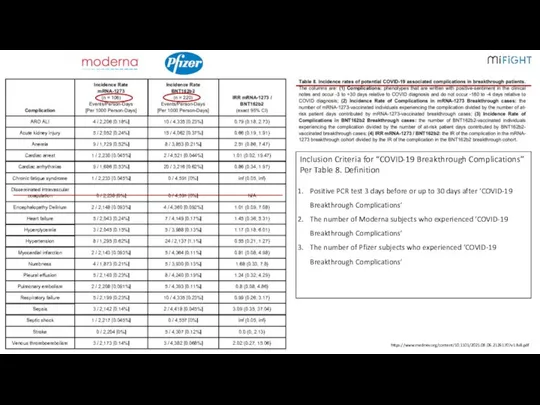
- 14. Pfizer/BioNTech vs Moderna Efficacy and “Breakthrough COVID-19” Cases MAYO Clinic – 5 States – 636,053 Subjects
- 15. 1 https://www.medrxiv.org/content/10.1101/2021.08.06.21261707v1.full.pdf
- 16. 1 https://www.medrxiv.org/content/10.1101/2021.08.06.21261707v1.full.pdf Inclusion Criteria for “COVID-19 Breakthrough Complications” Per Table 8. Definition Positive PCR test 3
- 18. Скачать презентацию












 Структура и задачи центрального процессора
Структура и задачи центрального процессора Книга рецептов
Книга рецептов итоговая аттестация
итоговая аттестация Фотоальбом
Фотоальбом Цифровые интегральные микросхемы
Цифровые интегральные микросхемы Презентация Microsoft PowerPoint
Презентация Microsoft PowerPoint Образец презентации 2 (Исторические)
Образец презентации 2 (Исторические) Վերջին զանգ
Վերջին զանգ Генезис философии и особенности мировоззренческой мысли на Древнем Востоке
Генезис философии и особенности мировоззренческой мысли на Древнем Востоке УралВагонЗавод
УралВагонЗавод Николай II 1 часть
Николай II 1 часть Сборка компьютера. (Глава 3)
Сборка компьютера. (Глава 3) Кандидат на роль старосты 5В класса МБОУ Гимназия №45
Кандидат на роль старосты 5В класса МБОУ Гимназия №45 Производственная практика ОАО ТОДЭП
Производственная практика ОАО ТОДЭП 20151117_distantsionnoe_obuchenie
20151117_distantsionnoe_obuchenie Мансардный этаж: проектирование и строительство
Мансардный этаж: проектирование и строительство Ar mums būvēt vienkārši un ērti
Ar mums būvēt vienkārši un ērti Святое безмолвие Христа
Святое безмолвие Христа Древесина. Строение древесины
Древесина. Строение древесины 20160128_poeziya_i_dissidentstvo_v_epohu_zastoya_chast_3
20160128_poeziya_i_dissidentstvo_v_epohu_zastoya_chast_3 Основы Иудейской Культуры
Основы Иудейской Культуры Открытое пространсво, архитектурное пространство, городская среда, городской интерьер
Открытое пространсво, архитектурное пространство, городская среда, городской интерьер Как научить детей с аутизмом считать
Как научить детей с аутизмом считать Образец оформления презентации проекта
Образец оформления презентации проекта кл предлоги
кл предлоги 3D печать. Преимущества, недостатки, перспективы развития
3D печать. Преимущества, недостатки, перспективы развития Як утворилися торф і кам'яне вугілля
Як утворилися торф і кам'яне вугілля Driverless car
Driverless car