Содержание
- 2. Outline: 1. Reservoir pressure and temperature 2.Reduced pressure and temperature 3.Physical and chemical properties of the
- 3. Reservoir pressure and temperature practice of using bottomhole pressure measurements to improve oil and gas production
- 5. Reservoir temperature is governed primarily by the reservoir’s proximity to the earth’s mantle, and by the
- 6. Reduced pressure and temperature Reduced pressure-the reduced pressure is defined as its actual pressure. P=P:Pc The
- 7. Physical and chemical properties of the oil under reservoir conditions Petroleum is one of the most
- 8. Crude oils in a natural reservoir under pressure contain dissolved natural gases which vaporize as the
- 9. The oil viscosity is measured as a function of pressure in most PVT laboratory measurements. The
- 10. Formation water and their physical properties Formation water exists naturally in the rock all along, before
- 11. The density and salinity Salinity and density share a positive relationship. As density increases, the amount
- 12. The compressibility of water. Water is essentially incompressible, especially under normal conditions. If you fill a
- 13. Oil and water saturation of reservoirs Hydrocarbon saturation is 1 (one) minus the water saturation. Most
- 14. Wetting and the capillary pressure. Wetting is the ability of a liquid to maintain contact with
- 15. Wetting
- 16. Wetting and the capillary pressure. The wetting phase is identified by its ability to preferentially diffuse
- 17. Capillary Pressure
- 18. Capillary pressure
- 20. Скачать презентацию
Outline:
1. Reservoir pressure and temperature
2.Reduced pressure and temperature
3.Physical and chemical properties
Outline:
1. Reservoir pressure and temperature
2.Reduced pressure and temperature
3.Physical and chemical properties
4.Shrinkage oil
5.Oil viscosity
6.Formation water and their physical properties
8.The density and salinity
9.The compressibility of water
10.Oil and water saturation of reservoirs
11.Wetting and the capillary pressure
Reservoir pressure and temperature
practice of using bottomhole pressure measurements to improve
Reservoir pressure and temperature
practice of using bottomhole pressure measurements to improve
The varied uses of bottomhole pressure and temperature measurements have increased in scope during the past two decades as instrumentation technologies have produced more reliable and accurate tools. These advances have made more applications possible, including multilayer reservoirs, horizontal wells, interference testing, and drawdown test interpretation
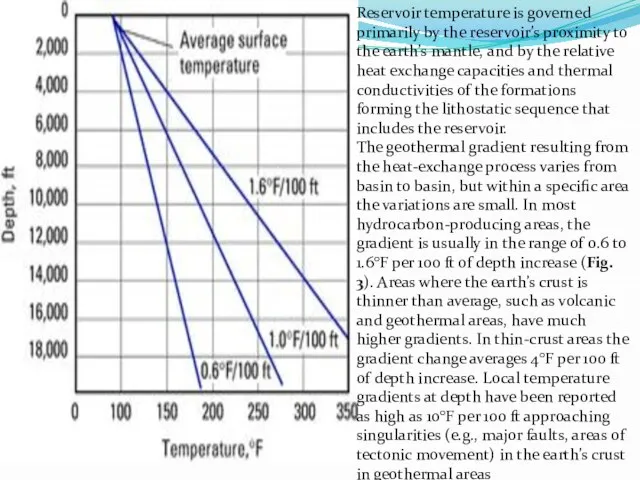
Reservoir temperature is governed primarily by the reservoir’s proximity to the
Reservoir temperature is governed primarily by the reservoir’s proximity to the
The geothermal gradient resulting from the heat-exchange process varies from basin to basin, but within a specific area the variations are small. In most hydrocarbon-producing areas, the gradient is usually in the range of 0.6 to 1.6°F per 100 ft of depth increase (Fig. 3). Areas where the earth’s crust is thinner than average, such as volcanic and geothermal areas, have much higher gradients. In thin-crust areas the gradient change averages 4°F per 100 ft of depth increase. Local temperature gradients at depth have been reported as high as 10°F per 100 ft approaching singularities (e.g., major faults, areas of tectonic movement) in the earth’s crust in geothermal areas
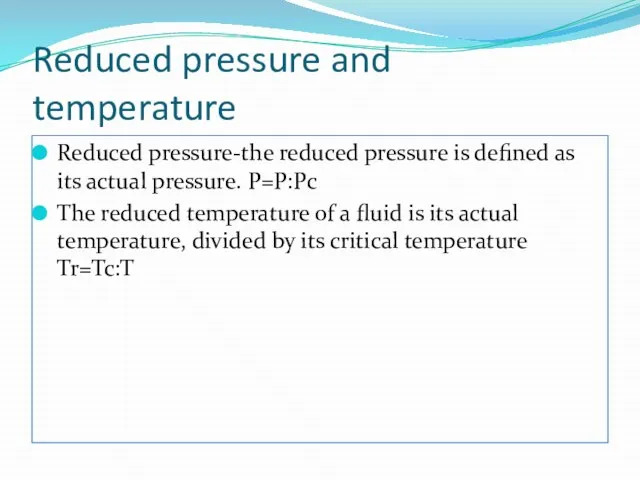
Reduced pressure and temperature
Reduced pressure-the reduced pressure is defined as its
Reduced pressure and temperature
Reduced pressure-the reduced pressure is defined as its
The reduced temperature of a fluid is its actual temperature, divided by its critical temperature Tr=Tc:T
Physical and chemical properties of the oil under reservoir conditions
Petroleum is
Physical and chemical properties of the oil under reservoir conditions
Petroleum is
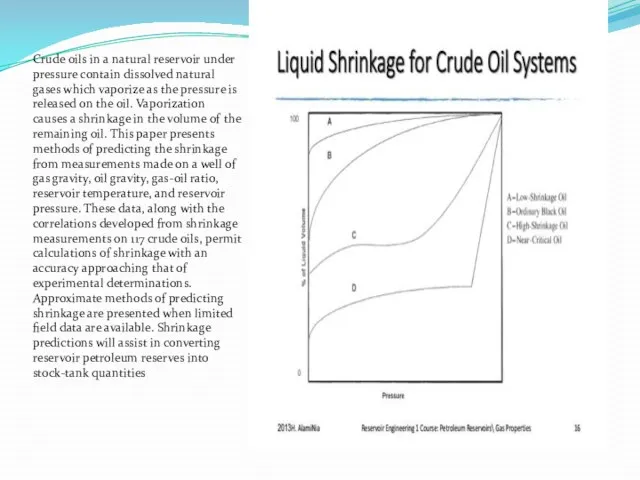
Crude oils in a natural reservoir under pressure contain dissolved natural
Crude oils in a natural reservoir under pressure contain dissolved natural
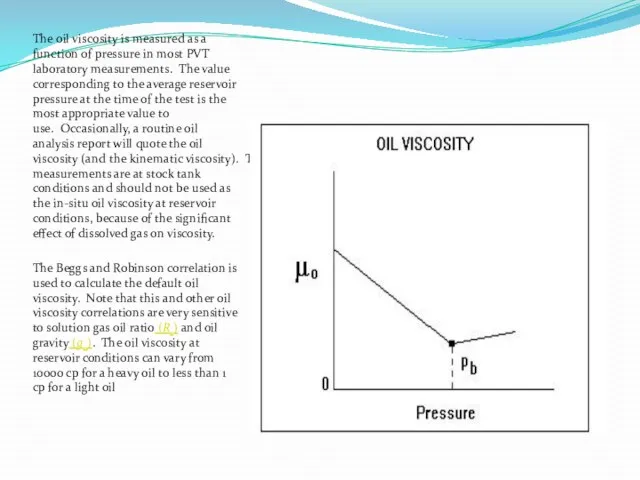
The oil viscosity is measured as a function of pressure in
The oil viscosity is measured as a function of pressure in
The Beggs and Robinson correlation is used to calculate the default oil viscosity. Note that this and other oil viscosity correlations are very sensitive to solution gas oil ratio (Rs) and oil gravity (go). The oil viscosity at reservoir conditions can vary from 10000 cp for a heavy oil to less than 1 cp for a light oil
Formation water and their physical properties
Formation water exists naturally in the
Formation water and their physical properties
Formation water exists naturally in the
The density and salinity
Salinity and density share a positive relationship. As
The density and salinity
Salinity and density share a positive relationship. As
The compressibility of water.
Water is essentially incompressible, especially under normal conditions.
The compressibility of water.
Water is essentially incompressible, especially under normal conditions.
Oil and water saturation of reservoirs
Hydrocarbon saturation is 1 (one) minus
Oil and water saturation of reservoirs
Hydrocarbon saturation is 1 (one) minus
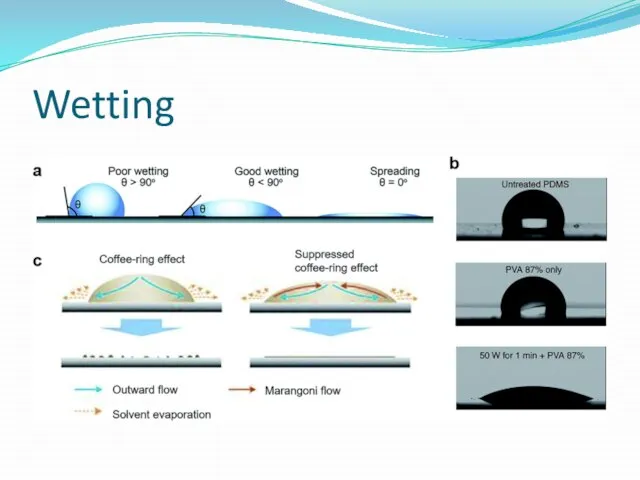
Wetting and the capillary pressure.
Wetting is the ability of a liquid
Wetting and the capillary pressure.
Wetting is the ability of a liquid
Wetting
Wetting
Wetting and the capillary pressure.
The wetting phase is identified by its
Wetting and the capillary pressure.
The wetting phase is identified by its
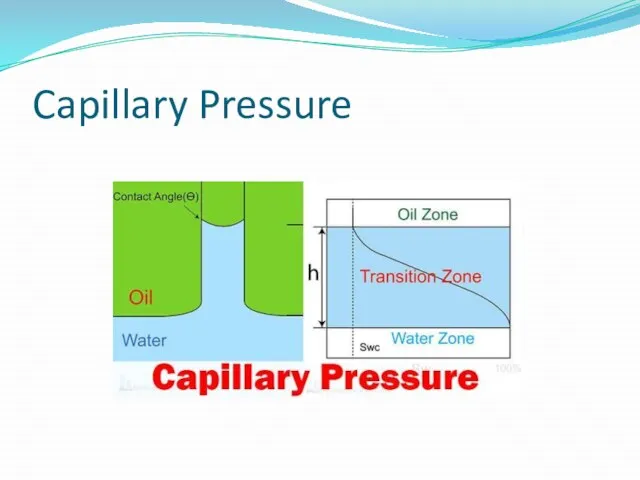
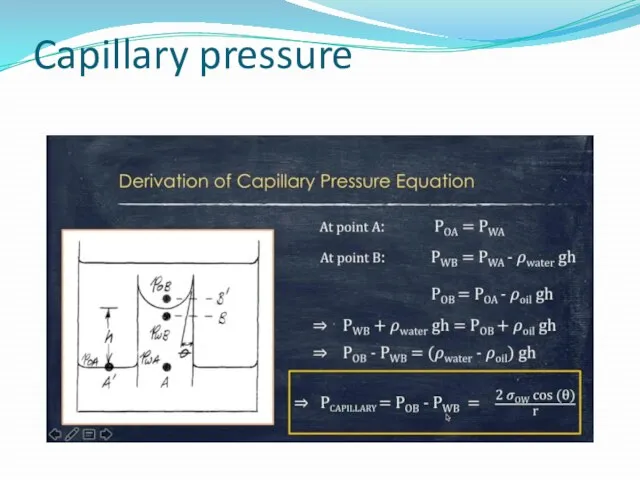
Capillary Pressure
Capillary Pressure
Capillary pressure
Capillary pressure








 Парогазовые установки с газификацией угля
Парогазовые установки с газификацией угля Современные пакеты прикладных программ статистической обработки информации
Современные пакеты прикладных программ статистической обработки информации 20130422_ahmatova_0
20130422_ahmatova_0 Sharing bicycle project
Sharing bicycle project Весна, любовь и комплименты
Весна, любовь и комплименты dr_gleblya
dr_gleblya ПРОБЛЕМЫ ТОЛЕРАНТНОСТИ В МИРОВОЙ ИСТОРИИ-ЛИТЕРАТУРА
ПРОБЛЕМЫ ТОЛЕРАНТНОСТИ В МИРОВОЙ ИСТОРИИ-ЛИТЕРАТУРА ИЗО_03.02
ИЗО_03.02 Определение в слове
Определение в слове Крапивина Л.А. Волонтёры Мы Вместе
Крапивина Л.А. Волонтёры Мы Вместе Домовенок
Домовенок 20120331_interaktivnaya_igra_4
20120331_interaktivnaya_igra_4 День-медицинского-работника
День-медицинского-работника Картина-портрет
Картина-портрет Интерьер
Интерьер Особенности построения изделий с рукавами рубашечного покроя
Особенности построения изделий с рукавами рубашечного покроя Мерки для построения чертежа плечевого изделия с цельнокроёным рукавом. Снятие мерок 7 кл
Мерки для построения чертежа плечевого изделия с цельнокроёным рукавом. Снятие мерок 7 кл Презентация Microsoft PowerPoint
Презентация Microsoft PowerPoint Шмаков Сергей Вячеславович. Резюме
Шмаков Сергей Вячеславович. Резюме Карьерный транспорт. Лекция 3. Карьерный железнодорожный транспорт
Карьерный транспорт. Лекция 3. Карьерный железнодорожный транспорт Кабельная сеть светофоров
Кабельная сеть светофоров Back rotary tines assembly
Back rotary tines assembly Технологические процессы заготовительных производств
Технологические процессы заготовительных производств 20151110_test_na_temu_iskusstvo
20151110_test_na_temu_iskusstvo Укрепление земляного полотна железной дороги с помощью Геосинтетических материалов
Укрепление земляного полотна железной дороги с помощью Геосинтетических материалов 20130220_1_klass_sadko
20130220_1_klass_sadko Железо - элемент цивилизации и жизни
Железо - элемент цивилизации и жизни Карьер по добыче песка ООО Каравелла
Карьер по добыче песка ООО Каравелла