Содержание
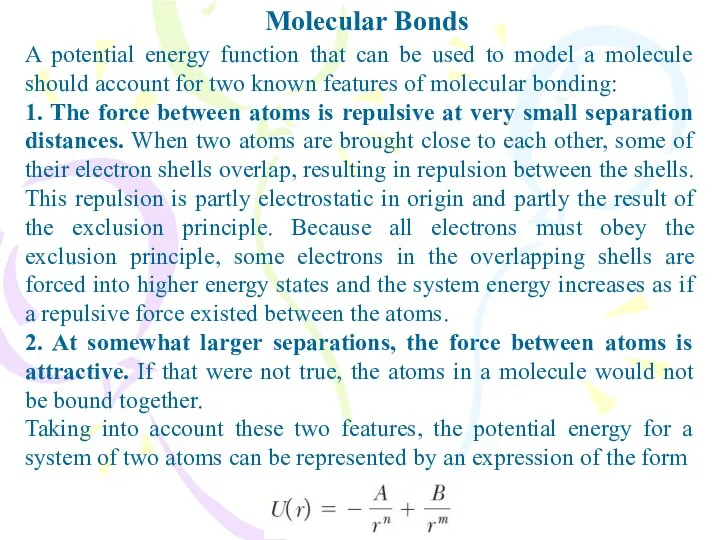
- 2. Molecular Bonds A potential energy function that can be used to model a molecule should account
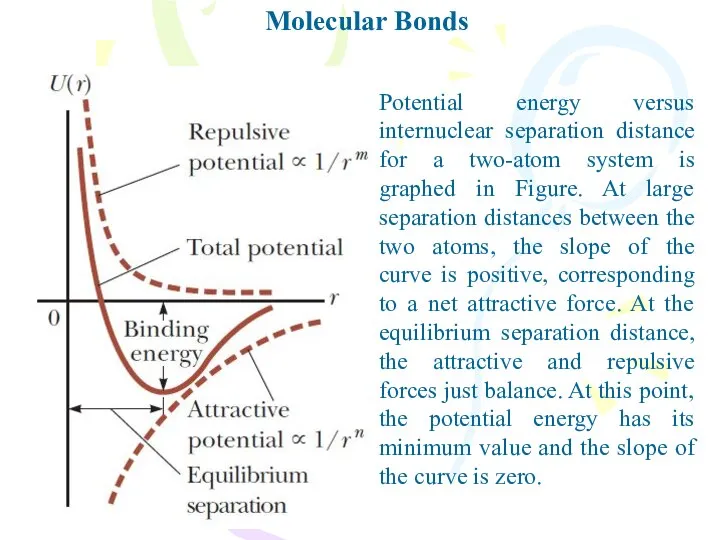
- 3. Molecular Bonds Potential energy versus internuclear separation distance for a two-atom system is graphed in Figure.
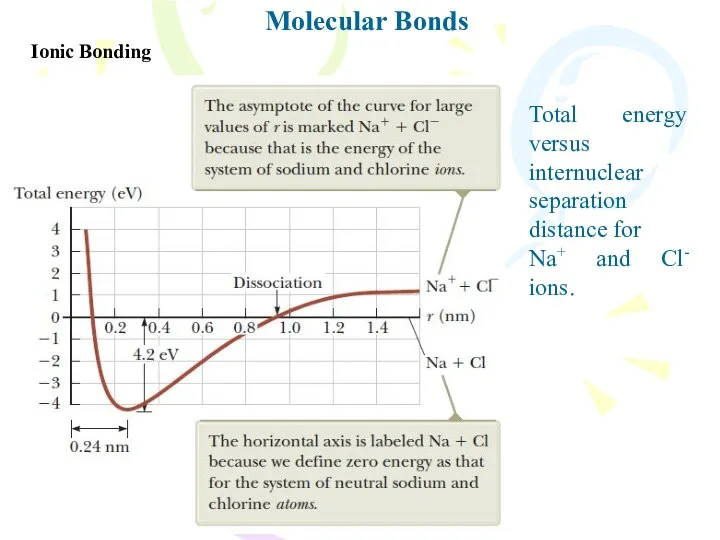
- 4. Molecular Bonds Ionic Bonding Total energy versus internuclear separation distance for Na+ and Cl- ions.
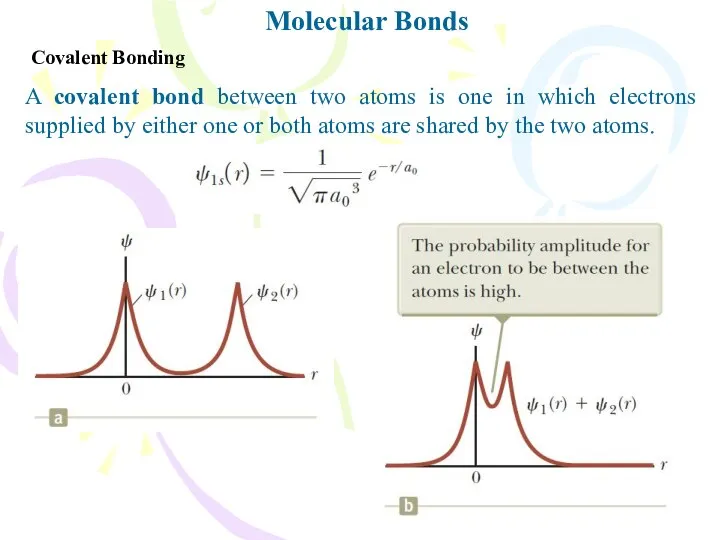
- 5. Molecular Bonds Covalent Bonding A covalent bond between two atoms is one in which electrons supplied
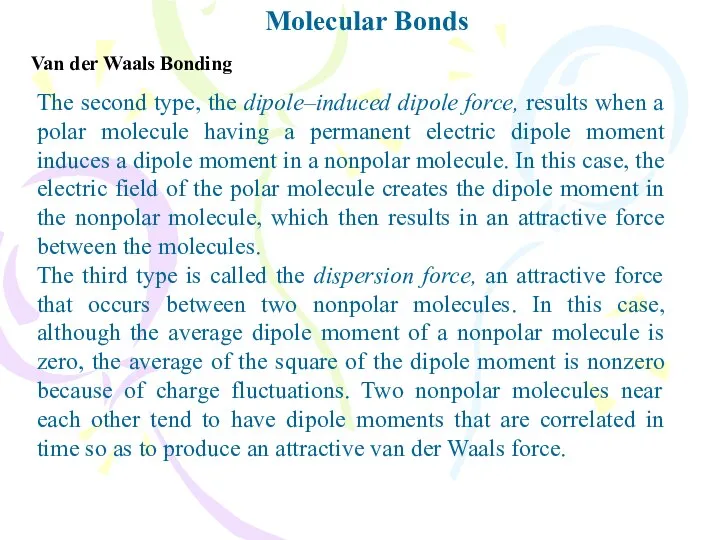
- 6. Molecular Bonds Van der Waals Bonding You might think that two neutral molecules would not interact
- 7. Molecular Bonds Van der Waals Bonding The second type, the dipole–induced dipole force, results when a
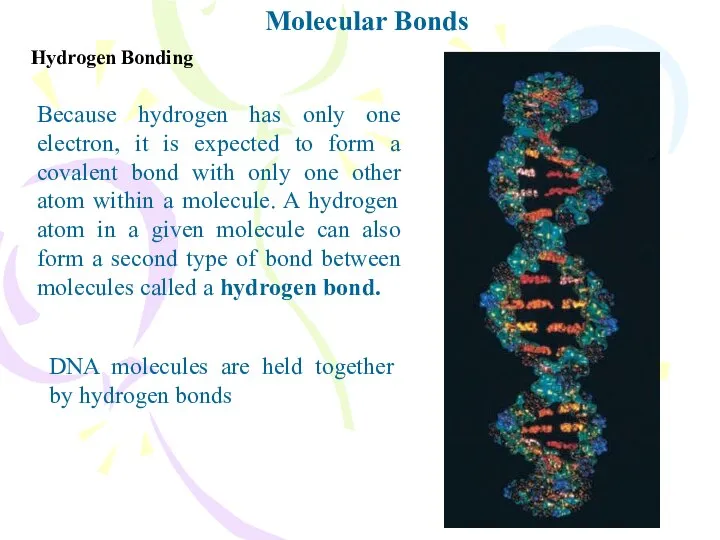
- 8. Molecular Bonds Hydrogen Bonding Because hydrogen has only one electron, it is expected to form a
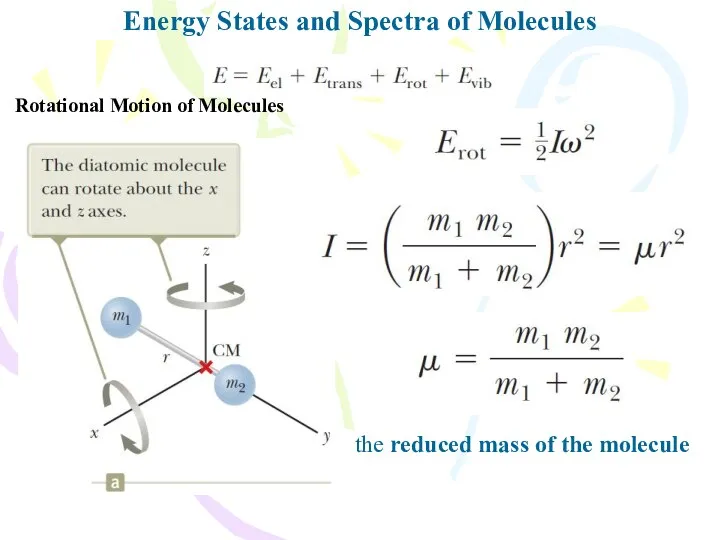
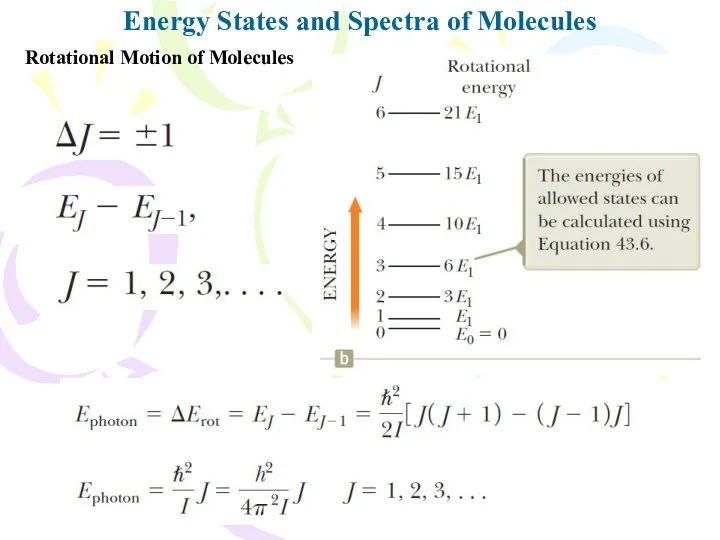
- 9. Energy States and Spectra of Molecules Rotational Motion of Molecules the reduced mass of the molecule
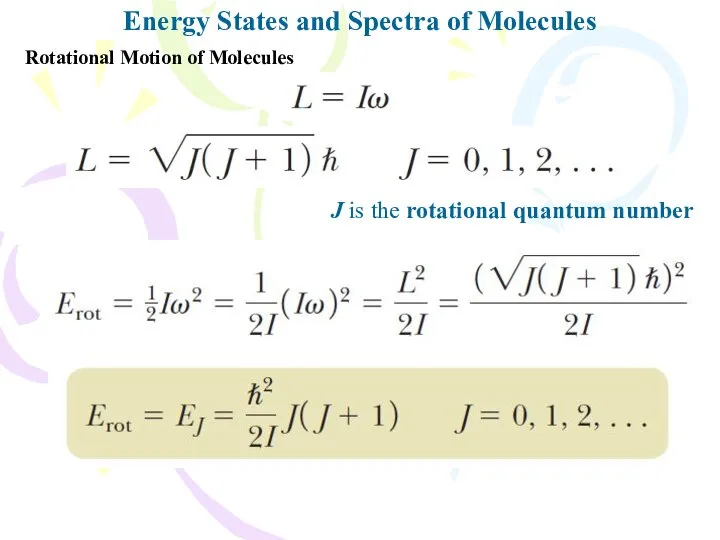
- 10. Energy States and Spectra of Molecules Rotational Motion of Molecules J is the rotational quantum number
- 11. Energy States and Spectra of Molecules Rotational Motion of Molecules
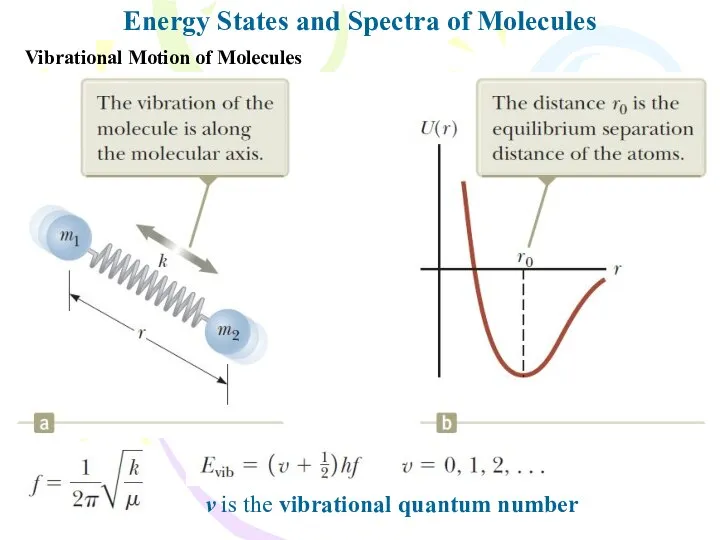
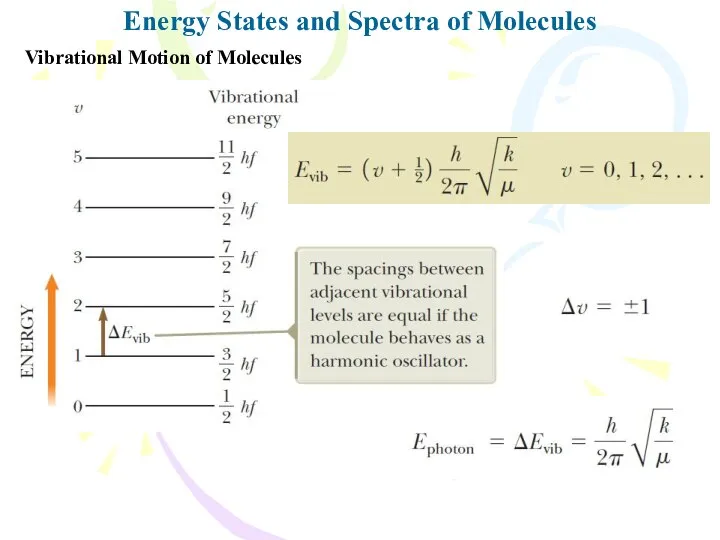
- 12. Energy States and Spectra of Molecules Vibrational Motion of Molecules v is the vibrational quantum number
- 13. Energy States and Spectra of Molecules Vibrational Motion of Molecules
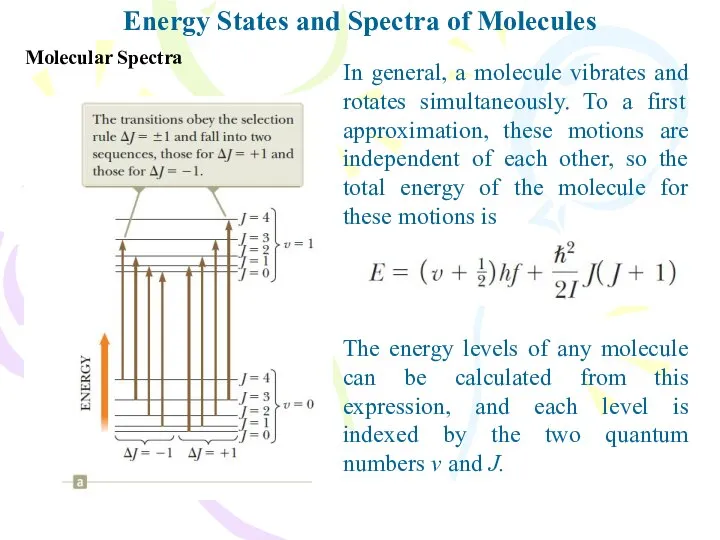
- 14. Energy States and Spectra of Molecules Molecular Spectra The energy levels of any molecule can be
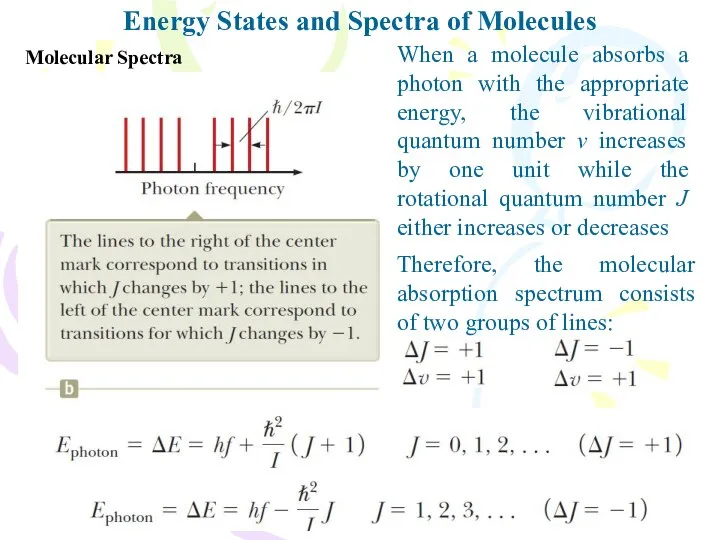
- 15. Molecular Spectra Energy States and Spectra of Molecules When a molecule absorbs a photon with the
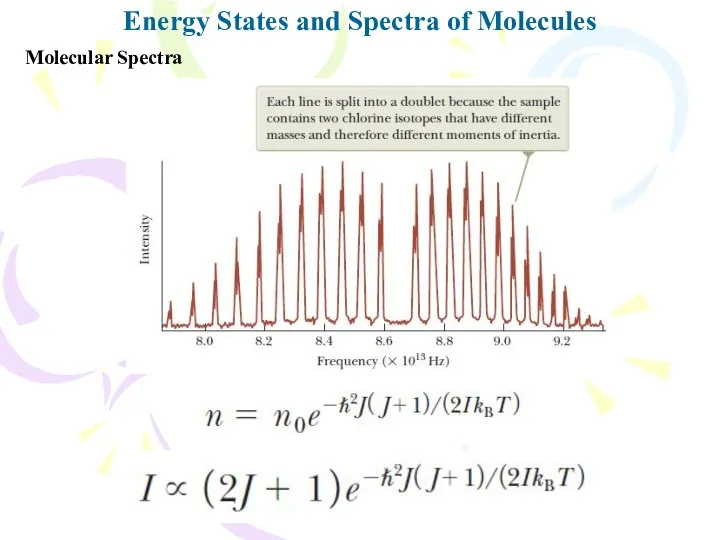
- 16. Molecular Spectra Energy States and Spectra of Molecules
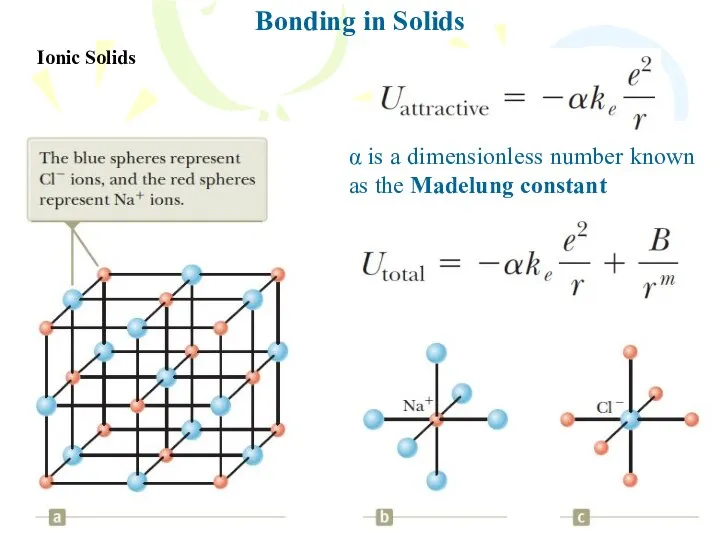
- 17. Bonding in Solids Ionic Solids α is a dimensionless number known as the Madelung constant
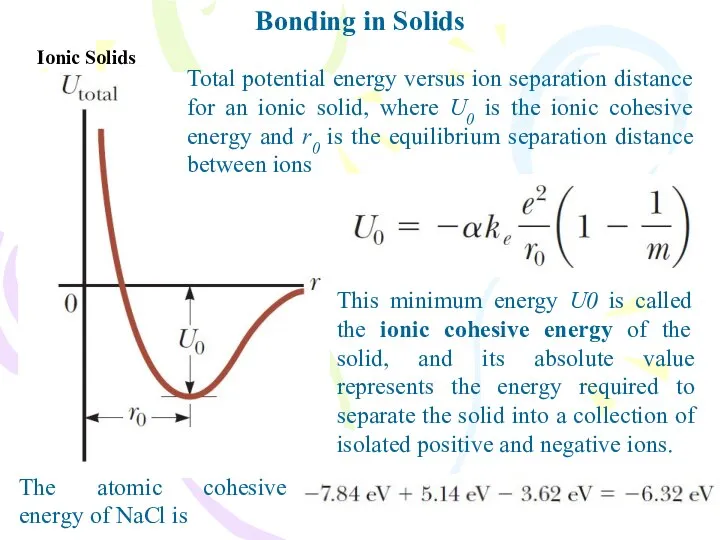
- 18. Bonding in Solids Ionic Solids Total potential energy versus ion separation distance for an ionic solid,
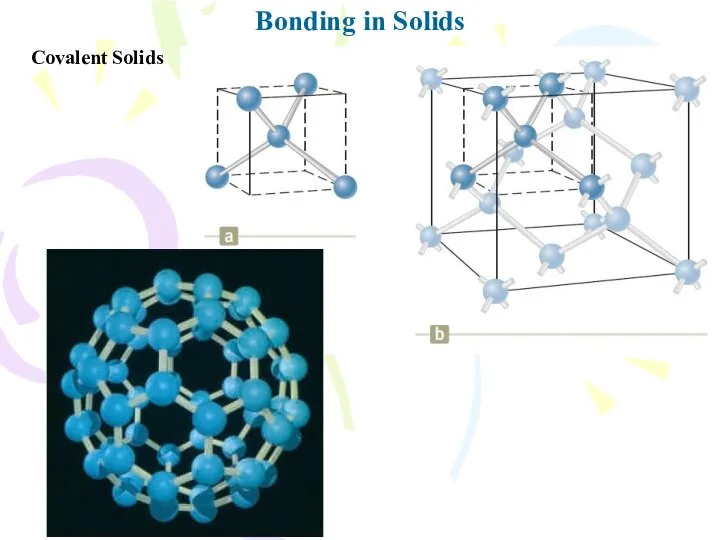
- 19. Bonding in Solids Covalent Solids
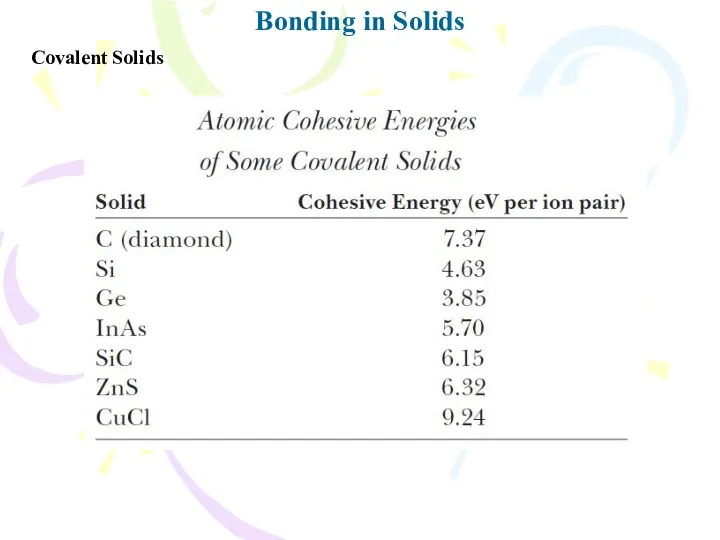
- 20. Bonding in Solids Covalent Solids
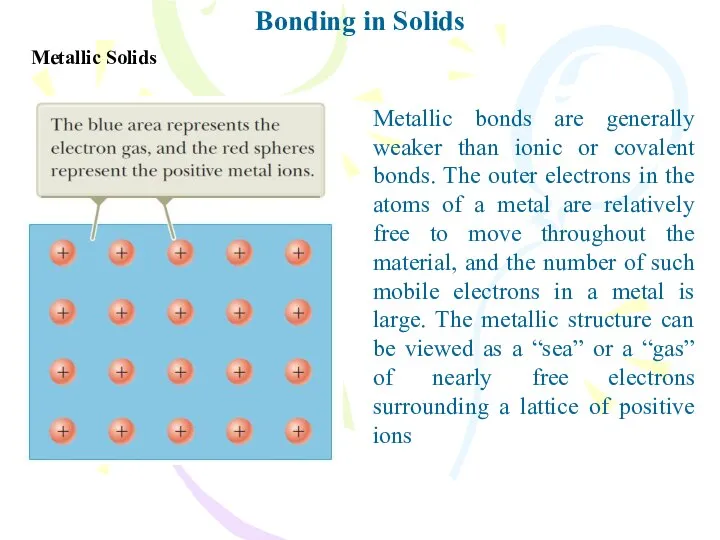
- 21. Bonding in Solids Metallic Solids Metallic bonds are generally weaker than ionic or covalent bonds. The
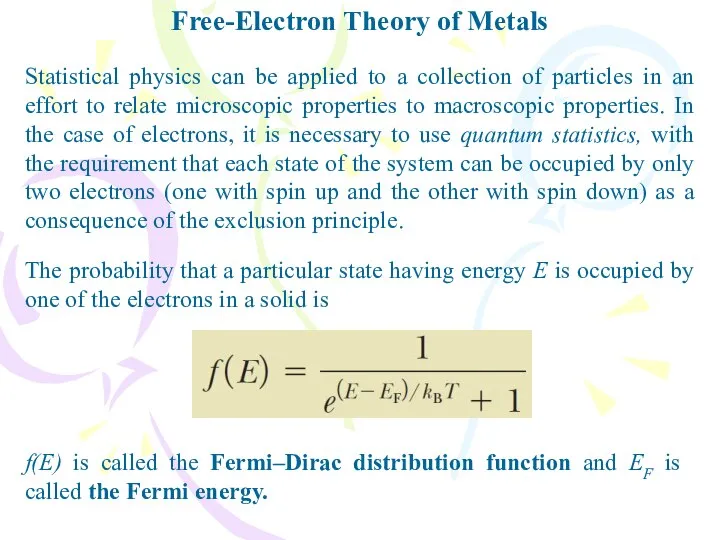
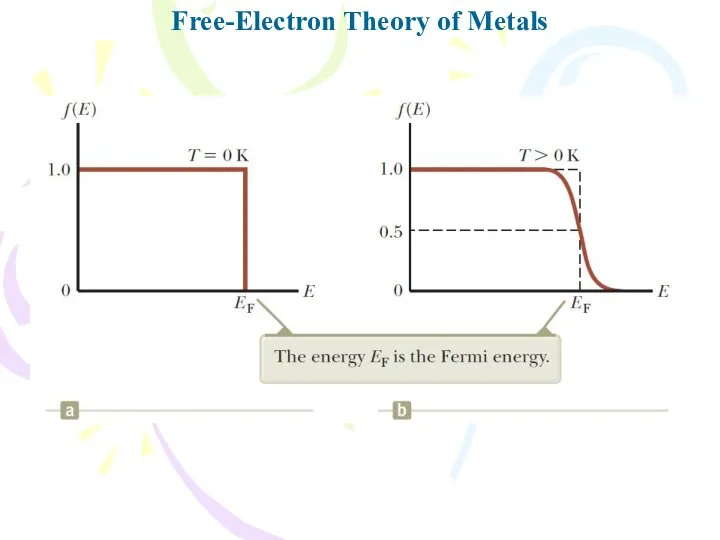
- 22. Free-Electron Theory of Metals The probability that a particular state having energy E is occupied by
- 23. Free-Electron Theory of Metals
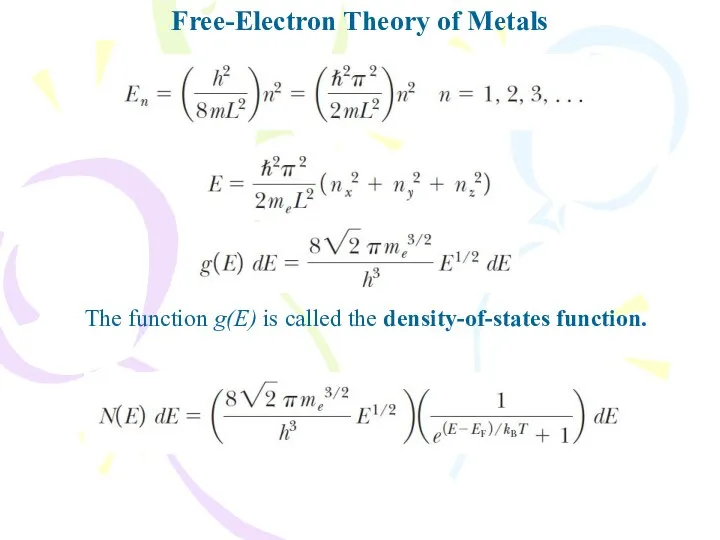
- 24. Free-Electron Theory of Metals The function g(E) is called the density-of-states function.
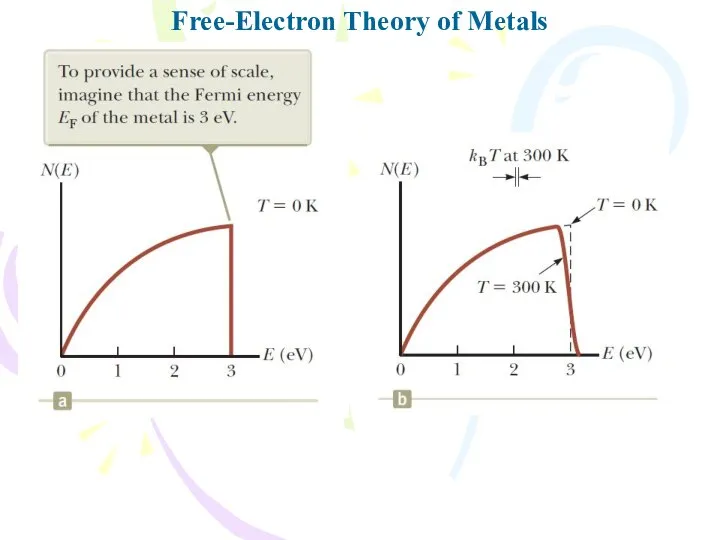
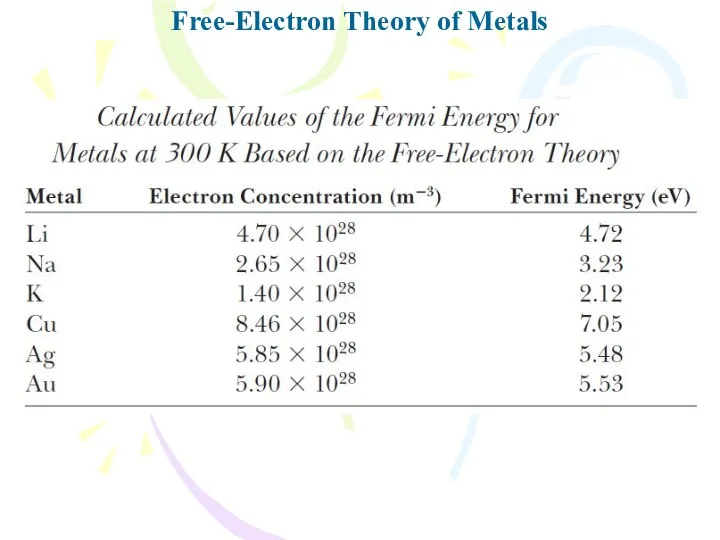
- 25. Free-Electron Theory of Metals
- 26. Free-Electron Theory of Metals
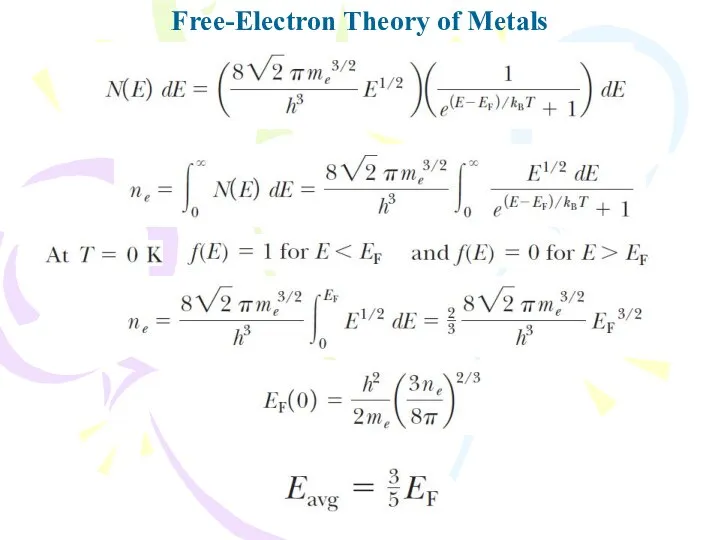
- 27. Free-Electron Theory of Metals
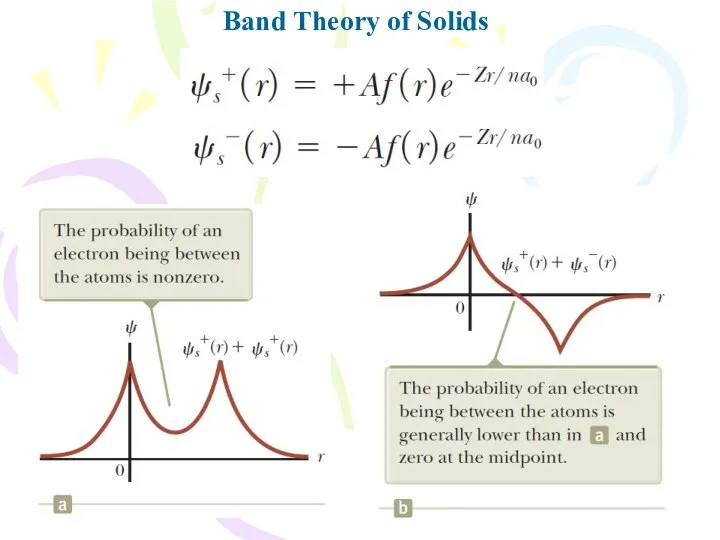
- 28. Band Theory of Solids
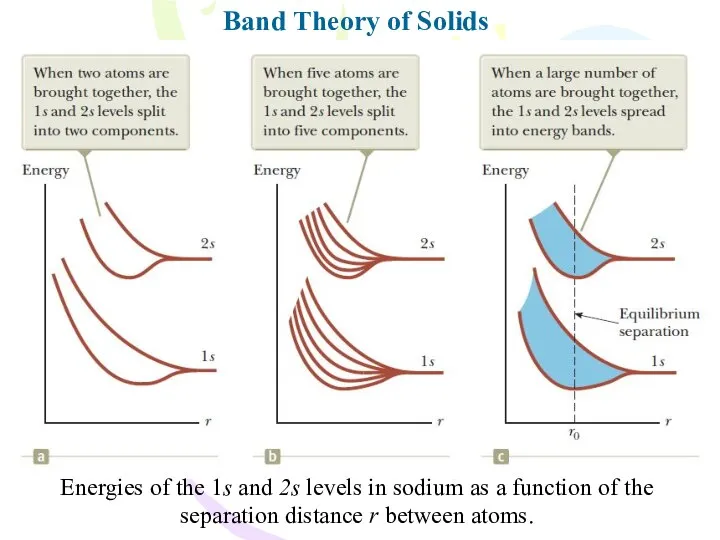
- 29. Band Theory of Solids Energies of the 1s and 2s levels in sodium as a function
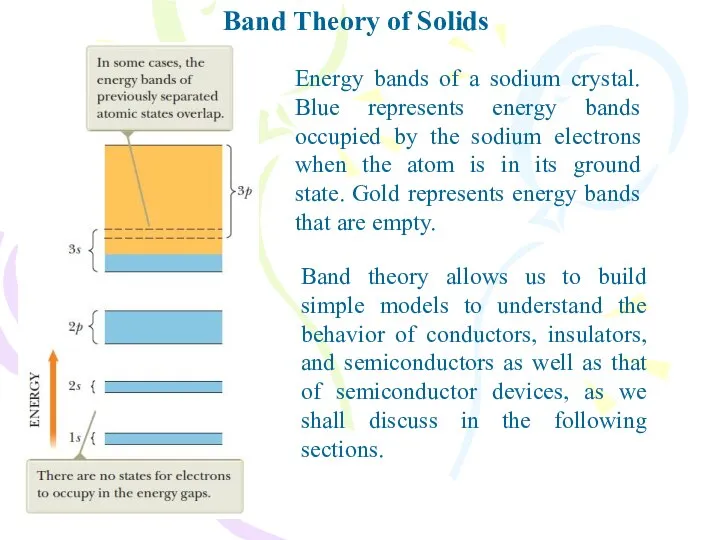
- 30. Band Theory of Solids Energy bands of a sodium crystal. Blue represents energy bands occupied by
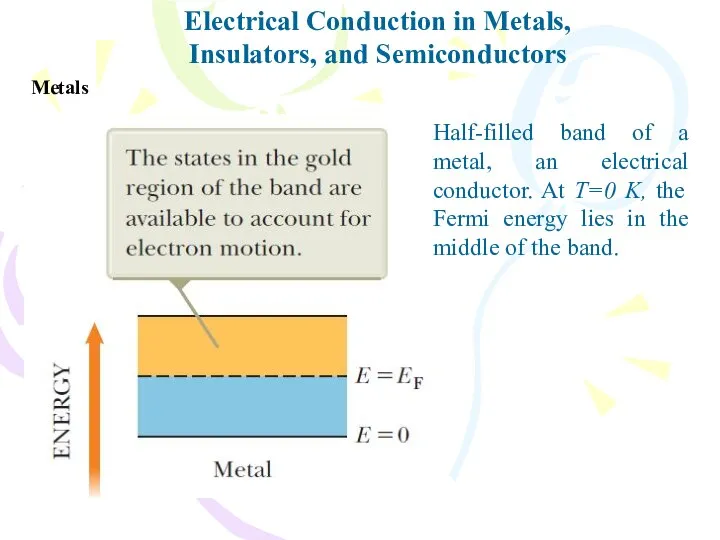
- 31. Electrical Conduction in Metals, Insulators, and Semiconductors Metals Half-filled band of a metal, an electrical conductor.
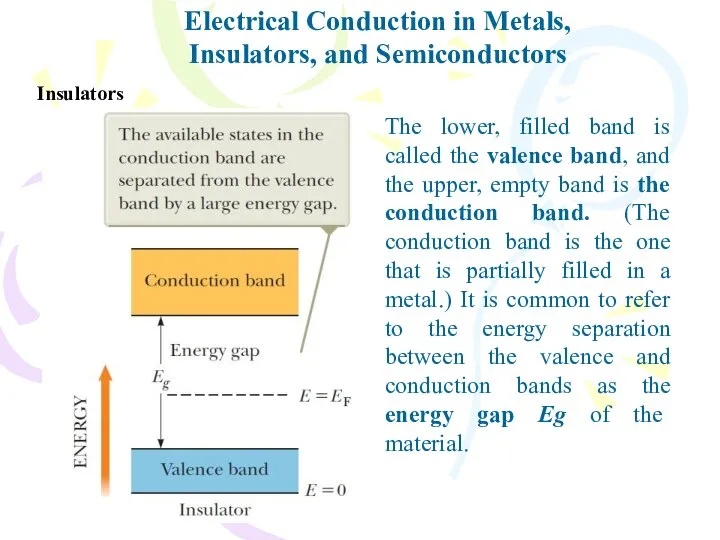
- 32. Electrical Conduction in Metals, Insulators, and Semiconductors Insulators The lower, filled band is called the valence
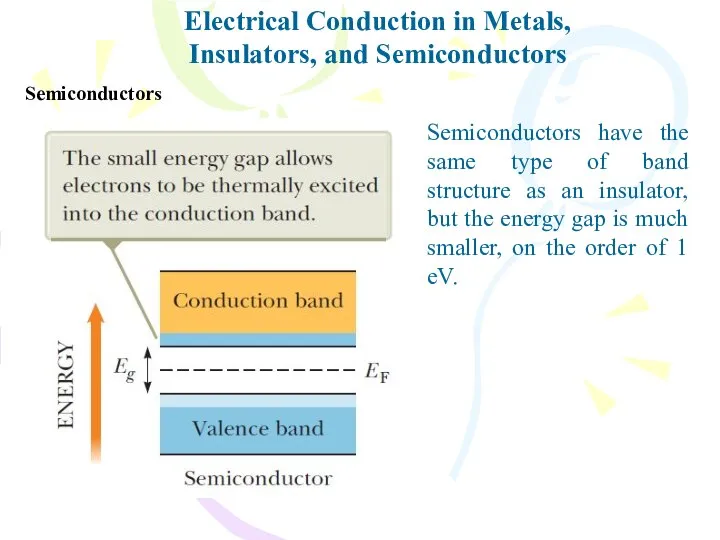
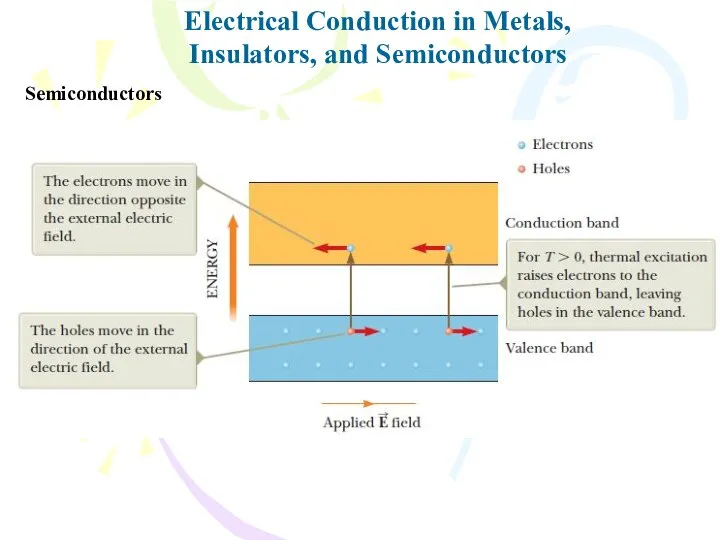
- 33. Electrical Conduction in Metals, Insulators, and Semiconductors Semiconductors Semiconductors have the same type of band structure
- 34. Electrical Conduction in Metals, Insulators, and Semiconductors Semiconductors
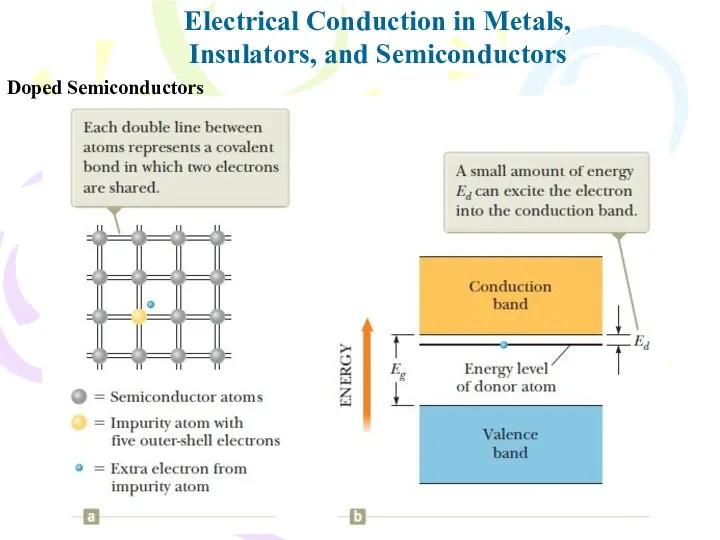
- 35. Doped Semiconductors Electrical Conduction in Metals, Insulators, and Semiconductors
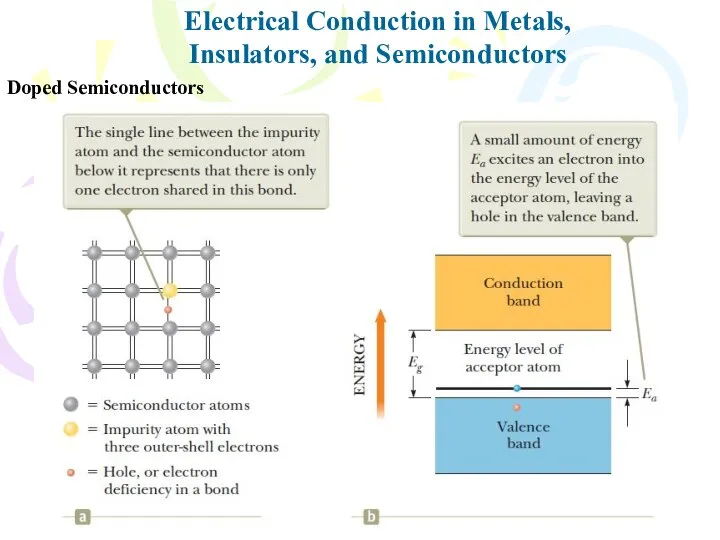
- 36. Electrical Conduction in Metals, Insulators, and Semiconductors Doped Semiconductors
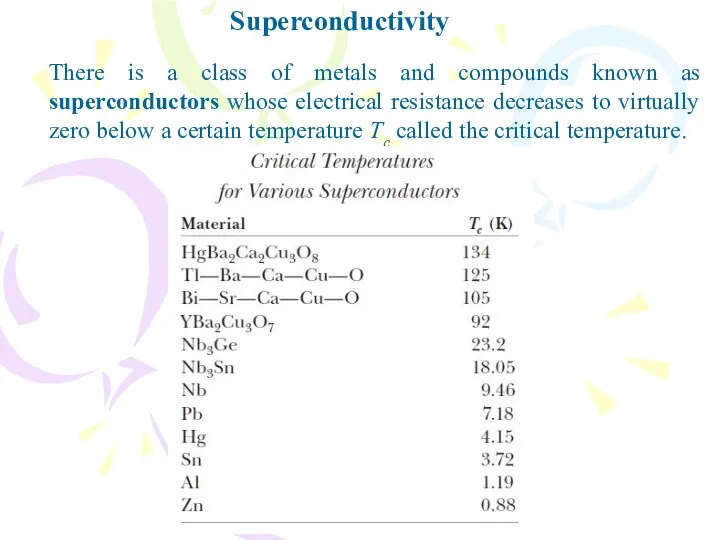
- 37. Superconductivity There is a class of metals and compounds known as superconductors whose electrical resistance decreases
- 39. Скачать презентацию

 C++ Ievade un izvade
C++ Ievade un izvade Федеральное агентство по образованию Российской Федерации Челябинский государственный университет Институт Экономики отраслей,
Федеральное агентство по образованию Российской Федерации Челябинский государственный университет Институт Экономики отраслей, Путешествие в город Математинск Автор: Цыбина Галина Яковлевна
Путешествие в город Математинск Автор: Цыбина Галина Яковлевна Физическое воспитание школьников и обучающихся в образовательных учреждениях до вузовского профессионального образования
Физическое воспитание школьников и обучающихся в образовательных учреждениях до вузовского профессионального образования Троица - Зелёные Святки
Троица - Зелёные Святки Метод комплексных амплитуд
Метод комплексных амплитуд Предмет теории и методики юношеского спорта
Предмет теории и методики юношеского спорта Введение в НТМL. Язык разметки гипертекста
Введение в НТМL. Язык разметки гипертекста Презентация "Человек и его украшения" - скачать презентации по МХК
Презентация "Человек и его украшения" - скачать презентации по МХК Сөйлемнің құрылымы
Сөйлемнің құрылымы «Мы первыми сумели на Земле открыть Вселенной запертые двери» 100 лет со дня рождения С.П.Королёва 150 лет со дня рождения К.Э. Циолко
«Мы первыми сумели на Земле открыть Вселенной запертые двери» 100 лет со дня рождения С.П.Королёва 150 лет со дня рождения К.Э. Циолко Коллективный договор
Коллективный договор Техника скандинавской ходьбы для каждого и в любое время
Техника скандинавской ходьбы для каждого и в любое время Окружающий мир 2 класс УМК «Начальная школа 21 века»
Окружающий мир 2 класс УМК «Начальная школа 21 века» Е.Д. Поливанов и его система транслитерации киридзи, как средство обучении японскому языку младших школьников
Е.Д. Поливанов и его система транслитерации киридзи, как средство обучении японскому языку младших школьников законы теплообмена
законы теплообмена  Беттік термомеханикалық өңдеу
Беттік термомеханикалық өңдеу Аттестационная работа. Элективный курс «Юный краевед»
Аттестационная работа. Элективный курс «Юный краевед» Слайд-презентации Тенденции и рекомендации
Слайд-презентации Тенденции и рекомендации Макеева Ольга Валентиновна, учитель математики МОУ гимназии №1 г. Липецка Математика – 6 Учебник – Н.Я.Виленкин, В.И.Жохов, А
Макеева Ольга Валентиновна, учитель математики МОУ гимназии №1 г. Липецка Математика – 6 Учебник – Н.Я.Виленкин, В.И.Жохов, А 7 класс
7 класс  Измерители механических напряжений гребного вала и счетчики топлива
Измерители механических напряжений гребного вала и счетчики топлива Комплект конструкторской документации на сборочную единицу
Комплект конструкторской документации на сборочную единицу Разработка функционирования модели ИС
Разработка функционирования модели ИС Как быстрее запомнить алфавит? Автор: Шестакова Евгения учащаяся 4 «а» класса МОУ СОШ №22
Как быстрее запомнить алфавит? Автор: Шестакова Евгения учащаяся 4 «а» класса МОУ СОШ №22 Владивостокский государственный университет экономики и сервиса Институт международного бизнеса и экономики Кафедра «Финанс
Владивостокский государственный университет экономики и сервиса Институт международного бизнеса и экономики Кафедра «Финанс Двухэтажный каттедж с мансардой в блокированной застройке
Двухэтажный каттедж с мансардой в блокированной застройке Художник и мир животных презентация к уроку изобразительного искусства 2 класс УМК «Гармония»
Художник и мир животных презентация к уроку изобразительного искусства 2 класс УМК «Гармония»