Содержание
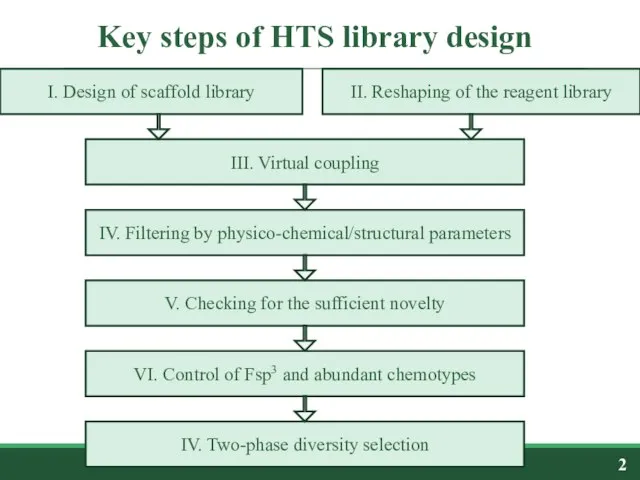
- 2. Key steps of HTS library design I. Design of scaffold library II. Reshaping of the reagent
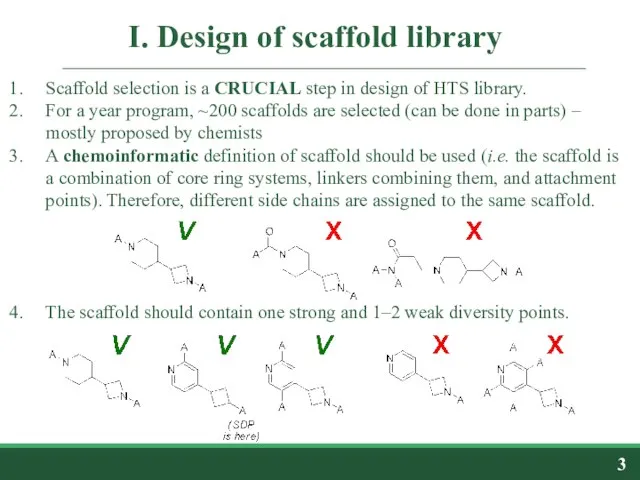
- 3. I. Design of scaffold library Scaffold selection is a CRUCIAL step in design of HTS library.
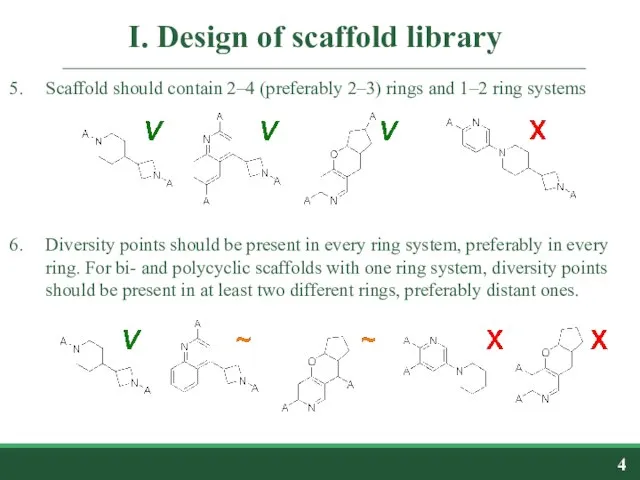
- 4. I. Design of scaffold library Scaffold should contain 2–4 (preferably 2–3) rings and 1–2 ring systems
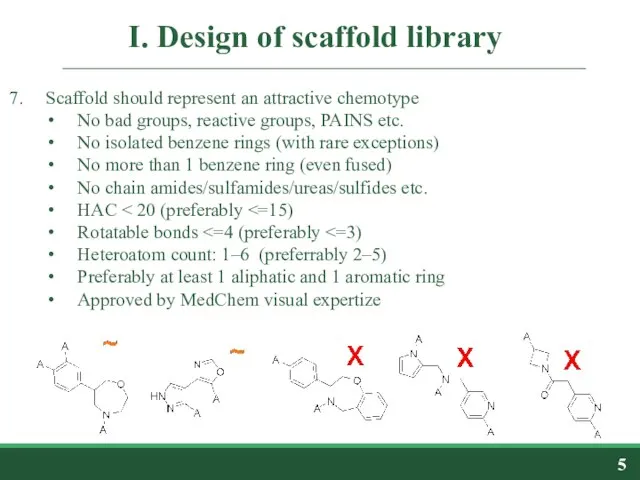
- 5. I. Design of scaffold library Scaffold should represent an attractive chemotype No bad groups, reactive groups,
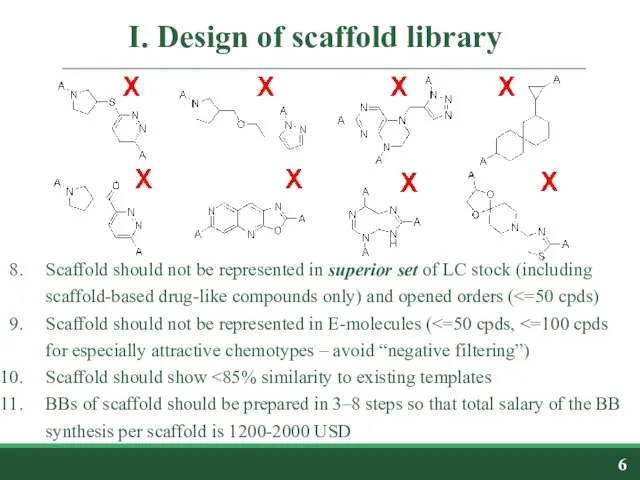
- 6. I. Design of scaffold library Scaffold should not be represented in superior set of LC stock
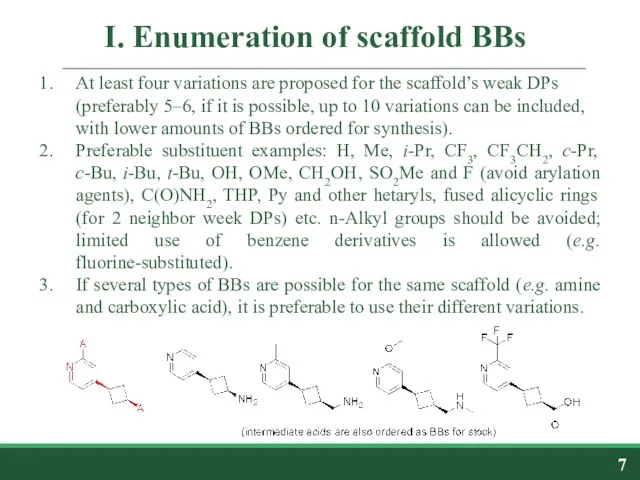
- 7. I. Enumeration of scaffold BBs At least four variations are proposed for the scaffold’s weak DPs
- 8. II. Reagent database A database of at least ~800–1000 appropriate reagents is needed for such a
- 9. IV. Filtering by PhysChem / Structure All available filters for bad, PAINS, reactive etc. (should not
- 10. V. Filtering by Novelty 98% Tanimoto diversity to competitors (E-molecules). 95% Tanimoto diversity to Advanced HTS
- 12. Скачать презентацию

 Метаболизм белков и аминокислот
Метаболизм белков и аминокислот Презентация на тему Аскариды, лечение аскаридоза у детей Общая информация об аскаридозе.
Презентация на тему Аскариды, лечение аскаридоза у детей Общая информация об аскаридозе. Что растёт на подоконнике?
Что растёт на подоконнике? Органы слуха
Органы слуха Презентация на тему "Значение бактерий в природе и жизни человека." - скачать бесплатно презентации по Биологии
Презентация на тему "Значение бактерий в природе и жизни человека." - скачать бесплатно презентации по Биологии Слуховой и вестибулярный анализаторы
Слуховой и вестибулярный анализаторы Колючий краб (Восточно-Сахалинская подзона)
Колючий краб (Восточно-Сахалинская подзона) Конференция на тему: «Молоко и молочные продукты» выполнили: ученики 3 «В» класса МОУ СОШ № 56 Минаев Роман, Копьев Алексей
Конференция на тему: «Молоко и молочные продукты» выполнили: ученики 3 «В» класса МОУ СОШ № 56 Минаев Роман, Копьев Алексей Возникновение земледелия. Лекция 2
Возникновение земледелия. Лекция 2 Эволюция дыхательной системы
Эволюция дыхательной системы Мышечная ткань
Мышечная ткань История представлений о возникновении жизни
История представлений о возникновении жизни Решение задач по биологии
Решение задач по биологии Список препаратов набор 1
Список препаратов набор 1 Самая курлыкающая птица. Серый журавль
Самая курлыкающая птица. Серый журавль Плоды
Плоды Движение крови и лимфы по сосудам
Движение крови и лимфы по сосудам Система дыхания
Система дыхания Строение и работа сердца
Строение и работа сердца  Эмбриологические доказательства эволюции
Эмбриологические доказательства эволюции Опорно-двигательный аппарат человека
Опорно-двигательный аппарат человека Эффективные средства по защите животных от эктопаразитов
Эффективные средства по защите животных от эктопаразитов Жизненный цикл человека
Жизненный цикл человека Презентация на тему "Прорастание семян" - скачать презентации по Биологии
Презентация на тему "Прорастание семян" - скачать презентации по Биологии Презентация на тему "Строение растительной клетки на примере клеток кожицы чешуи лука" - скачать презентации по Биологии
Презентация на тему "Строение растительной клетки на примере клеток кожицы чешуи лука" - скачать презентации по Биологии Тема урока: Генетика пола и наследование, сцепленное с полом Цель урока: изучить сущность хромосомного определения пола, механи
Тема урока: Генетика пола и наследование, сцепленное с полом Цель урока: изучить сущность хромосомного определения пола, механи Презентация по биологии Отряд хищные
Презентация по биологии Отряд хищные  Вплив різних видів фізичної активності на діяльність серця. Регуляція діяльності серця при фізичному навантаженні
Вплив різних видів фізичної активності на діяльність серця. Регуляція діяльності серця при фізичному навантаженні