Содержание
- 2. INTRODUCTION This Powerpoint show is one of several produced to help students understand selected topics at
- 3. CONTENTS Introduction Chemical and physical bonding Ionic bonding Covalent bonding Simple molecules Van der Waals’ forces
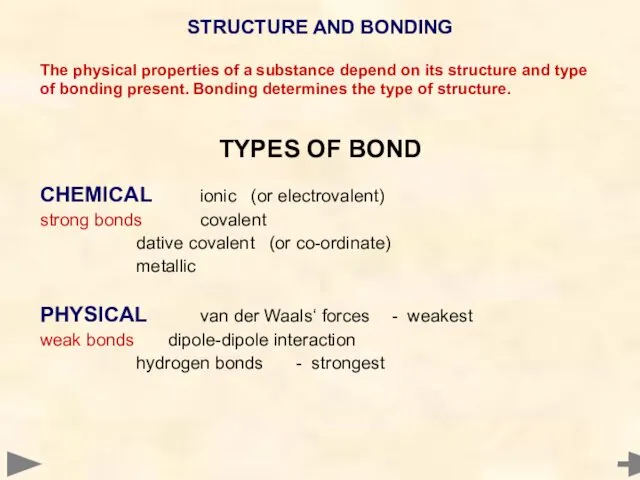
- 4. STRUCTURE AND BONDING The physical properties of a substance depend on its structure and type of
- 5. STRUCTURE AND BONDING The physical properties of a substance depend on its structure and type of
- 6. IONIC BONDING
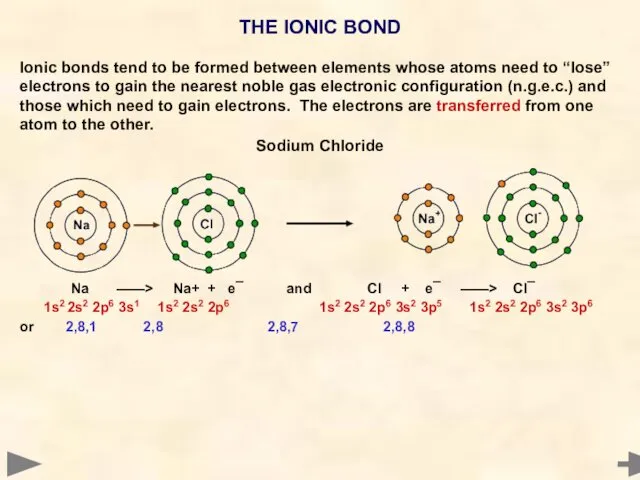
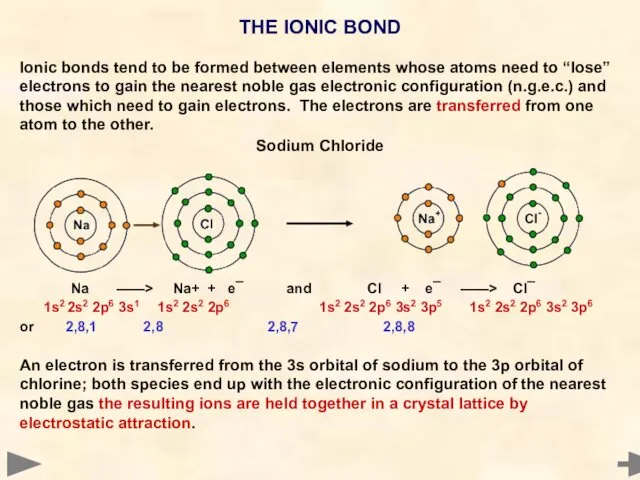
- 7. THE IONIC BOND Ionic bonds tend to be formed between elements whose atoms need to “lose”
- 8. THE IONIC BOND Ionic bonds tend to be formed between elements whose atoms need to “lose”
- 9. THE IONIC BOND Ionic bonds tend to be formed between elements whose atoms need to “lose”
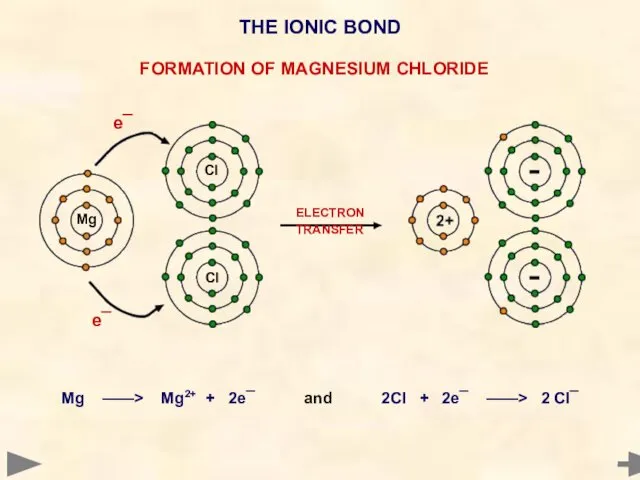
- 10. ELECTRON TRANSFER Mg ——> Mg2+ + 2e¯ and 2Cl + 2e¯ ——> 2 Cl¯ Mg Cl
- 11. Positive ions also known as cations; they are smaller than the original atom. formed when electrons
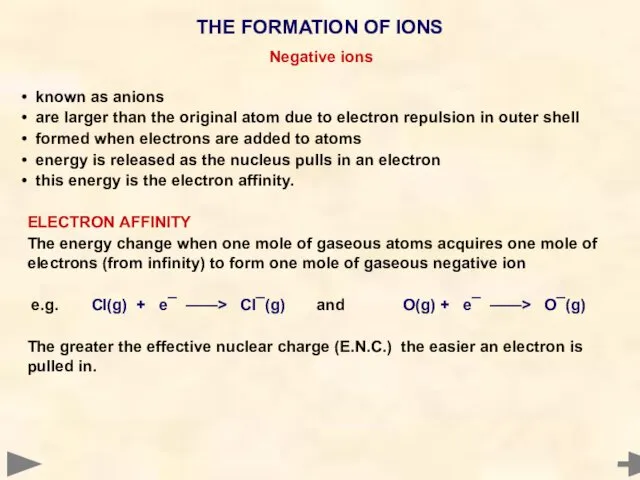
- 12. Negative ions known as anions are larger than the original atom due to electron repulsion in
- 13. IONIC BONDING Animations
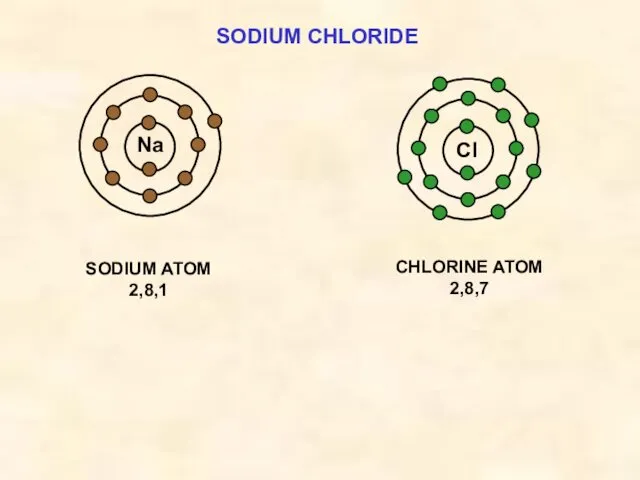
- 14. SODIUM CHLORIDE Cl SODIUM ATOM 2,8,1 Na CHLORINE ATOM 2,8,7
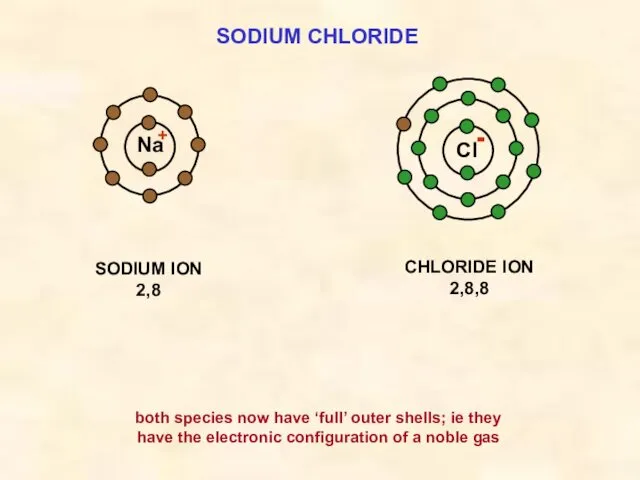
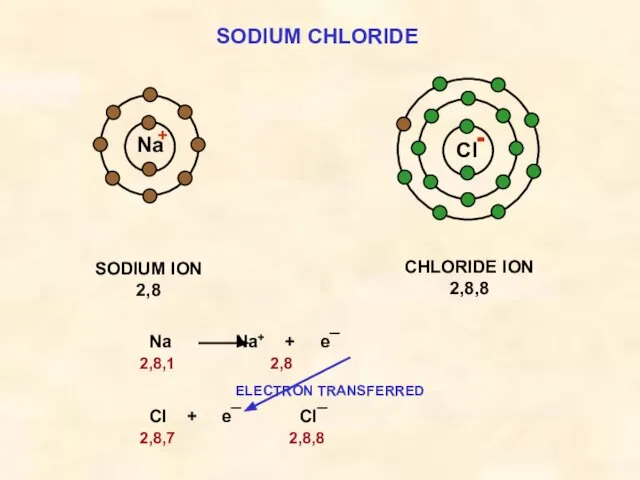
- 15. SODIUM CHLORIDE Cl SODIUM ION 2,8 Na CHLORIDE ION 2,8,8 both species now have ‘full’ outer
- 16. SODIUM CHLORIDE Cl SODIUM ION 2,8 Na CHLORIDE ION 2,8,8 Na Na+ + e¯ 2,8,1 2,8
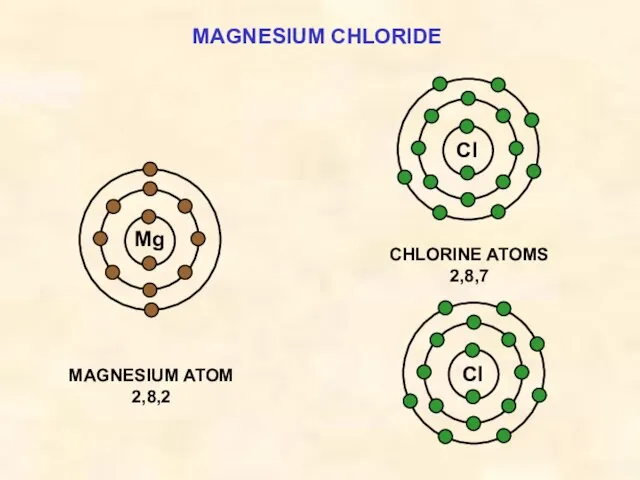
- 17. MAGNESIUM CHLORIDE Cl MAGNESIUM ATOM 2,8,2 Mg CHLORINE ATOMS 2,8,7 Cl
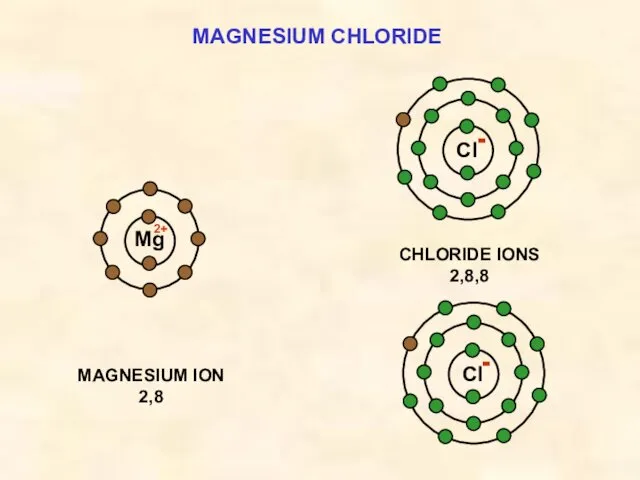
- 18. MAGNESIUM CHLORIDE Cl MAGNESIUM ION 2,8 Mg CHLORIDE IONS 2,8,8 Cl 2+
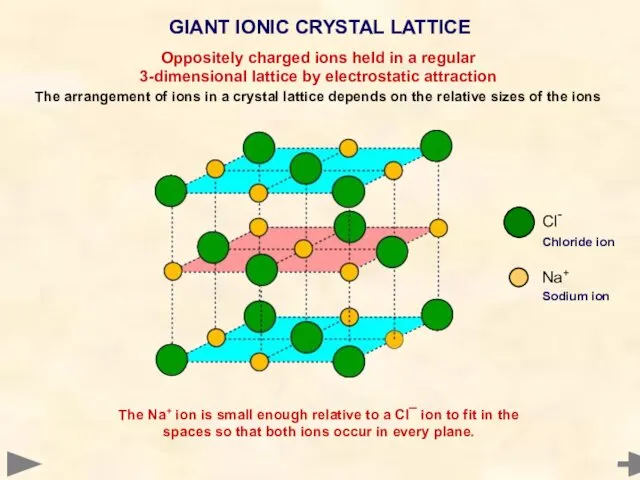
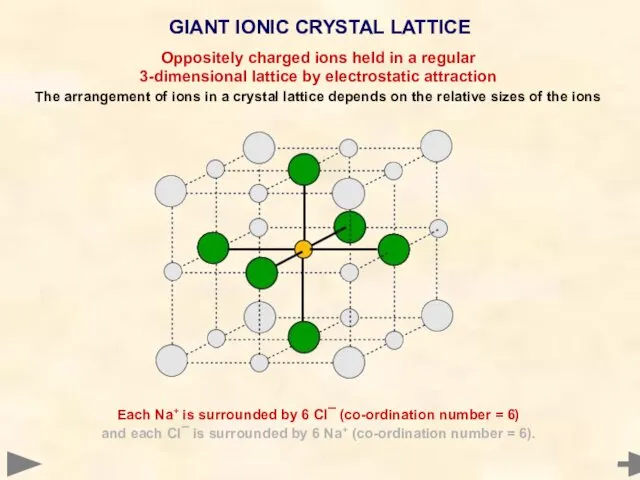
- 19. GIANT IONIC CRYSTAL LATTICE Cl- Chloride ion Na+ Sodium ion Oppositely charged ions held in a
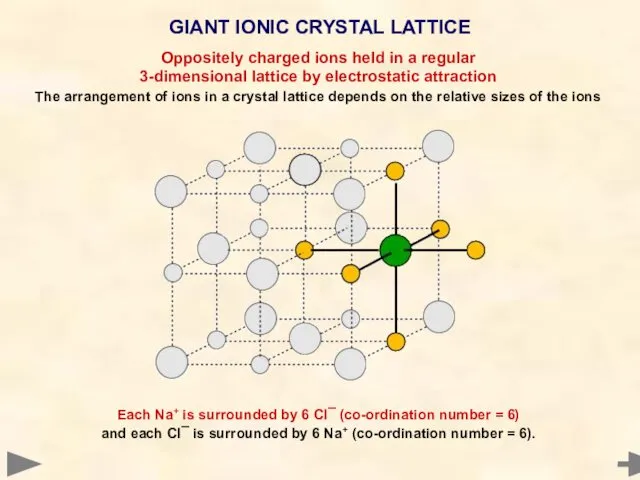
- 20. GIANT IONIC CRYSTAL LATTICE Each Na+ is surrounded by 6 Cl¯ (co-ordination number = 6) and
- 21. GIANT IONIC CRYSTAL LATTICE Each Na+ is surrounded by 6 Cl¯ (co-ordination number = 6) and
- 22. Physical properties of ionic compounds Melting point very high A large amount of energy must be
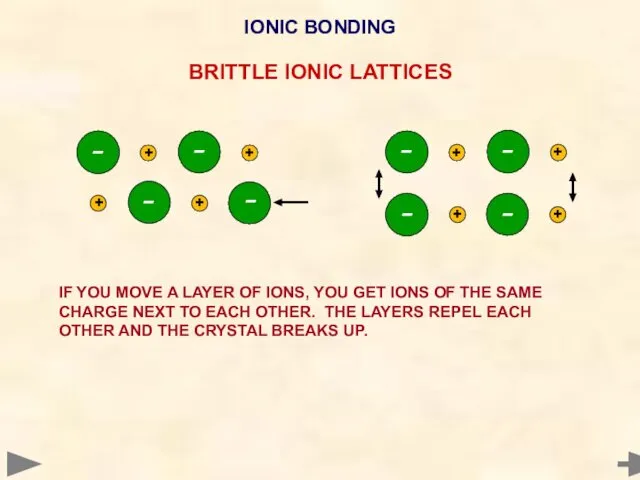
- 23. IONIC BONDING BRITTLE IONIC LATTICES IF YOU MOVE A LAYER OF IONS, YOU GET IONS OF
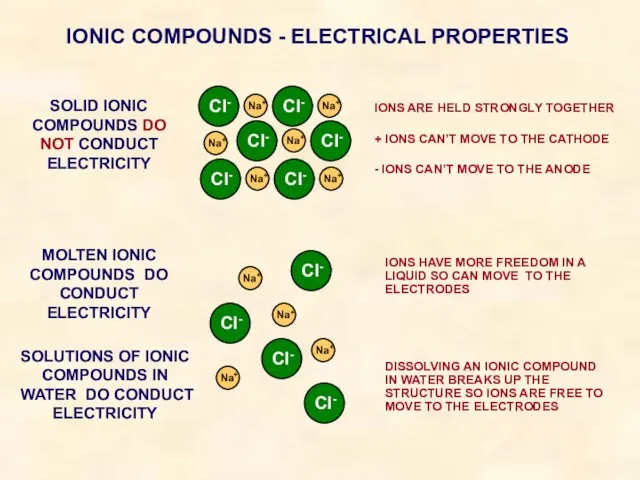
- 24. IONIC COMPOUNDS - ELECTRICAL PROPERTIES SOLID IONIC COMPOUNDS DO NOT CONDUCT ELECTRICITY IONS ARE HELD STRONGLY
- 25. COVALENT BONDING
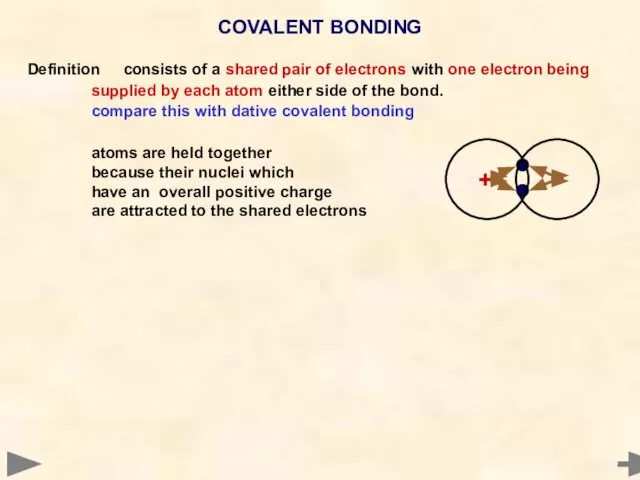
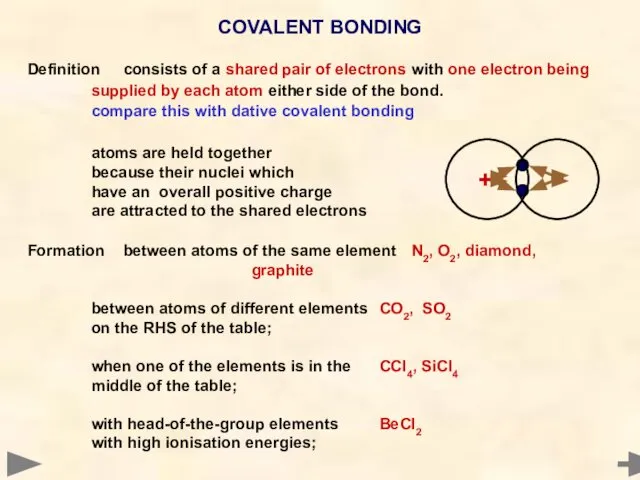
- 26. Definition consists of a shared pair of electrons with one electron being supplied by each atom
- 27. Definition consists of a shared pair of electrons with one electron being supplied by each atom
- 28. • atoms share electrons to get the nearest noble gas electronic configuration • some don’t achieve
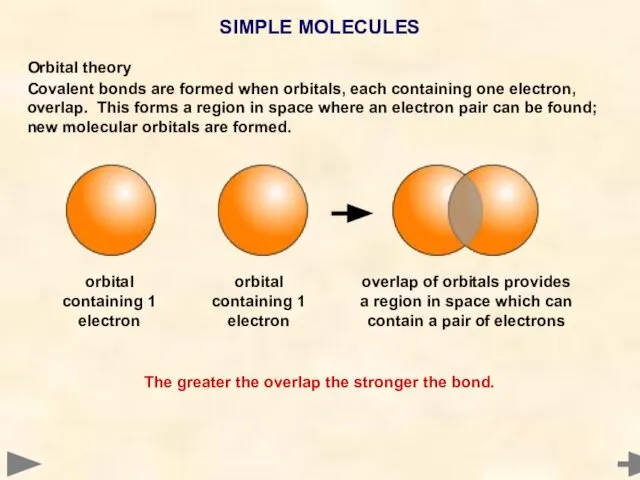
- 29. Orbital theory Covalent bonds are formed when orbitals, each containing one electron, overlap. This forms a
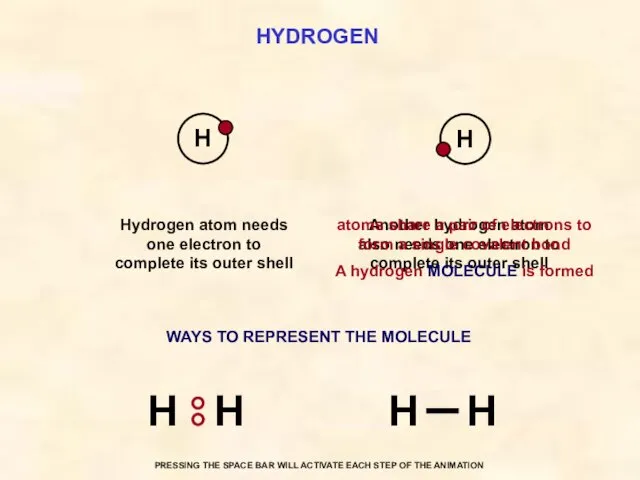
- 30. HYDROGEN Another hydrogen atom also needs one electron to complete its outer shell Hydrogen atom needs
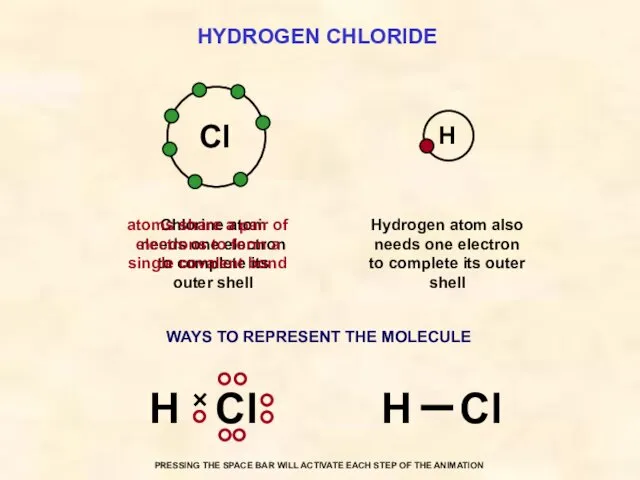
- 31. HYDROGEN CHLORIDE Cl Hydrogen atom also needs one electron to complete its outer shell Chlorine atom
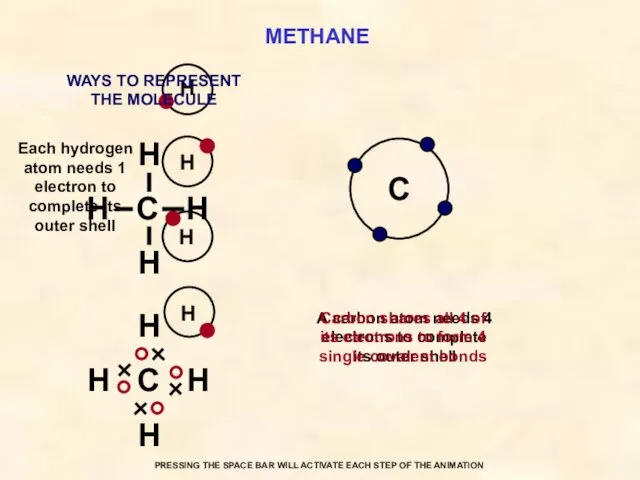
- 32. METHANE C Each hydrogen atom needs 1 electron to complete its outer shell A carbon atom
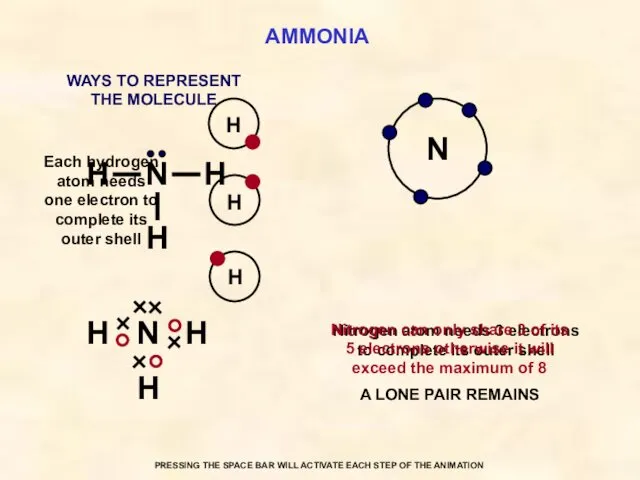
- 33. AMMONIA N Each hydrogen atom needs one electron to complete its outer shell Nitrogen atom needs
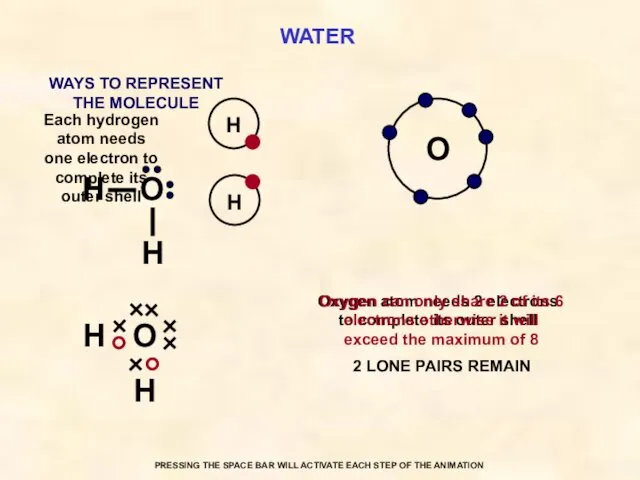
- 34. WATER O Each hydrogen atom needs one electron to complete its outer shell Oxygen atom needs
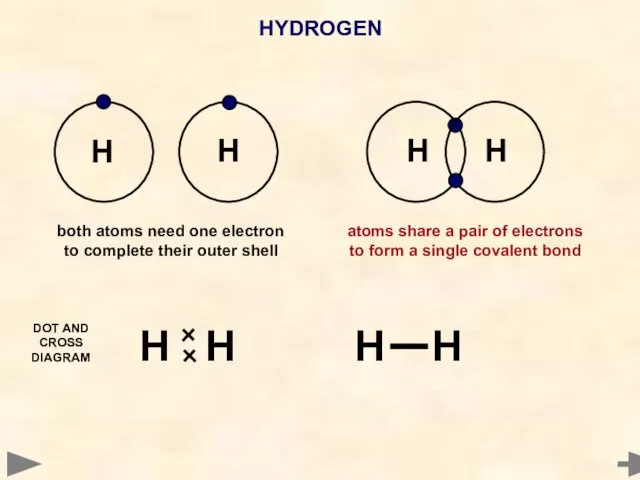
- 35. HYDROGEN H H H H H H H H both atoms need one electron to complete
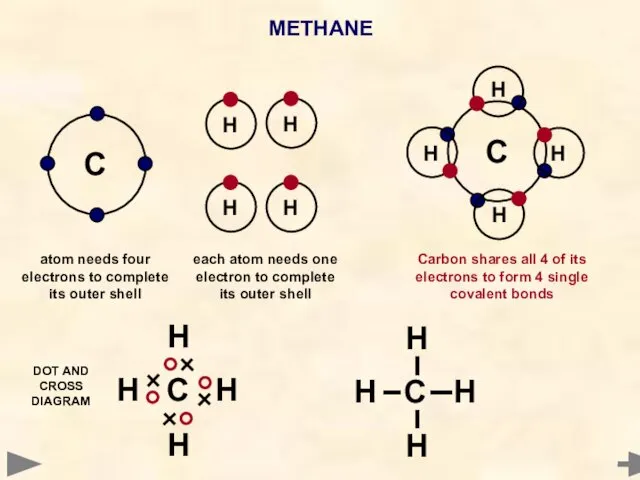
- 36. METHANE C H H H H C H H H H H C H H H
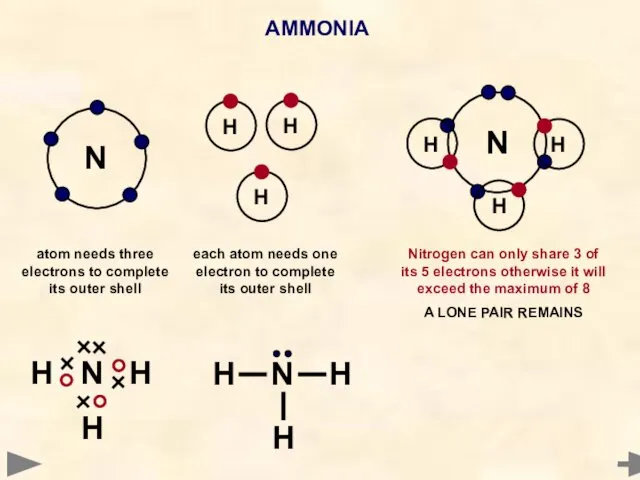
- 37. AMMONIA N H H H N H H H H N H H each atom needs
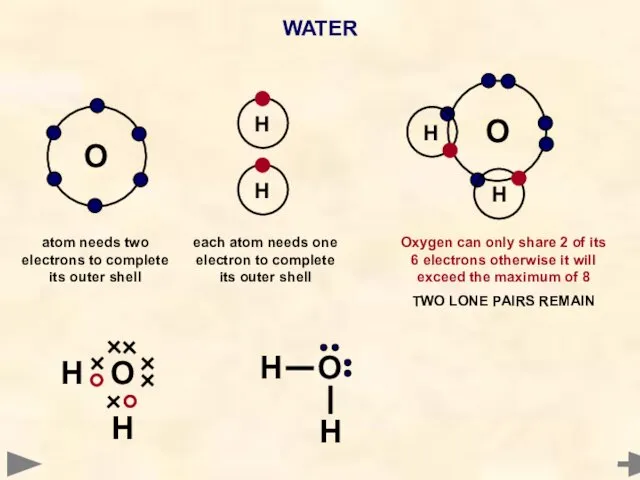
- 38. WATER O H H O H H each atom needs one electron to complete its outer
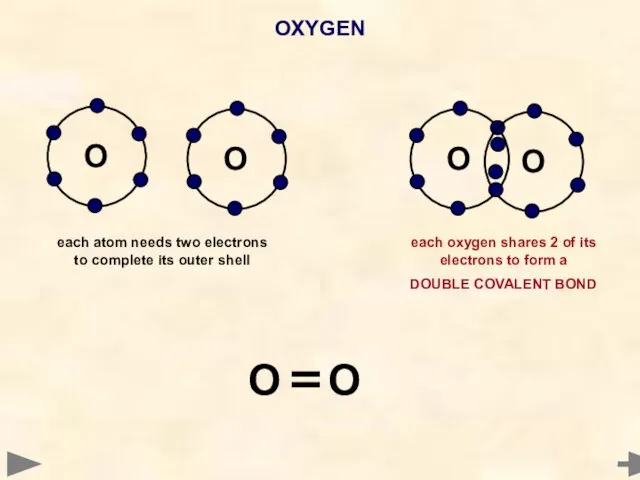
- 39. OXYGEN O each atom needs two electrons to complete its outer shell each oxygen shares 2
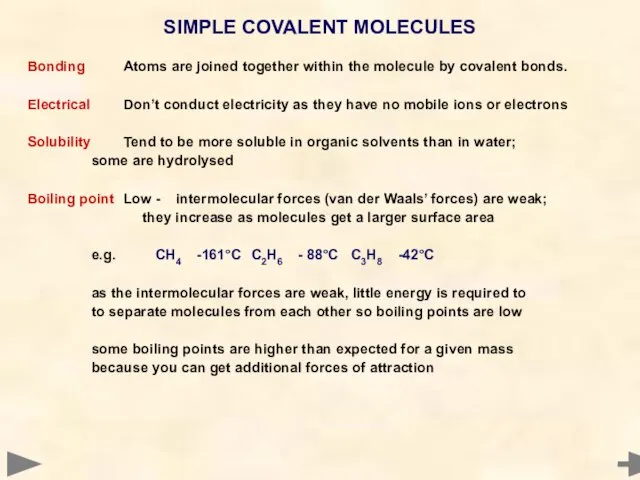
- 40. Bonding Atoms are joined together within the molecule by covalent bonds. Electrical Don’t conduct electricity as
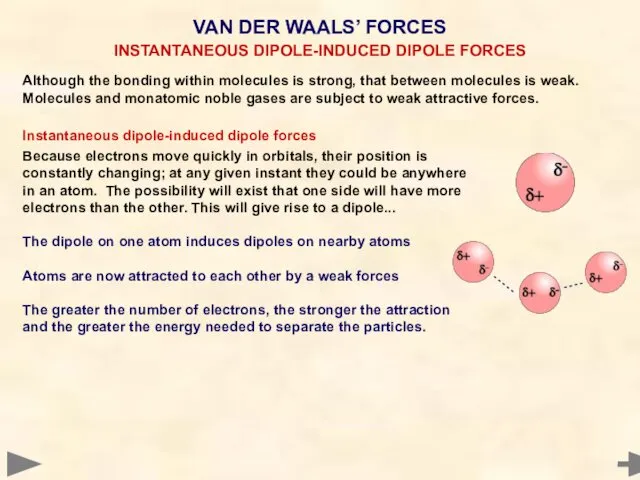
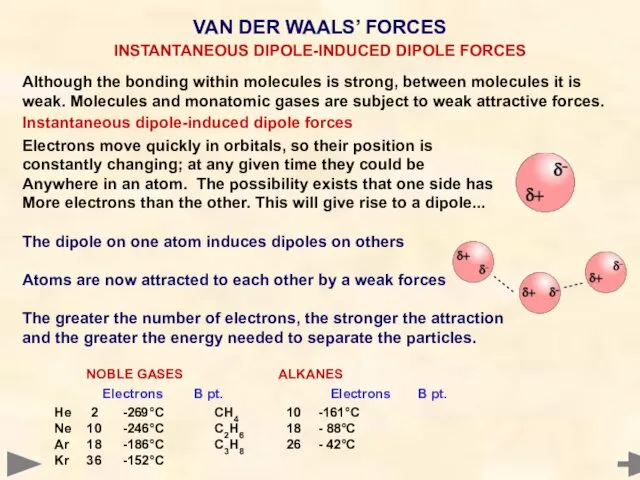
- 41. Although the bonding within molecules is strong, that between molecules is weak. Molecules and monatomic noble
- 42. Although the bonding within molecules is strong, that between molecules is weak. Molecules and monatomic noble
- 43. Although the bonding within molecules is strong, between molecules it is weak. Molecules and monatomic gases
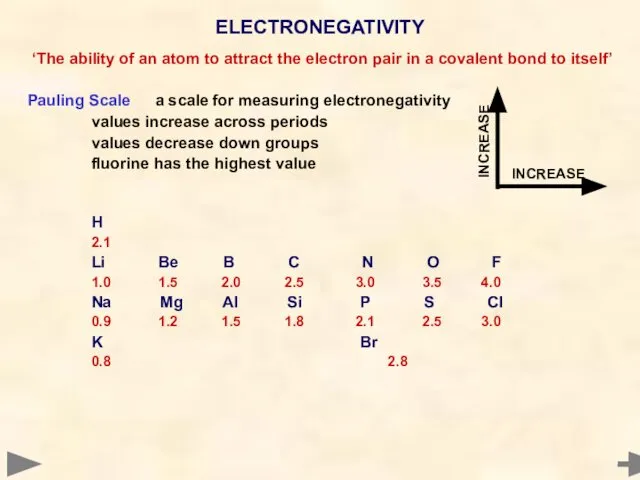
- 44. ‘The ability of an atom to attract the electron pair in a covalent bond to itself’
- 45. ‘The ability of an atom to attract the electron pair in a covalent bond to itself’
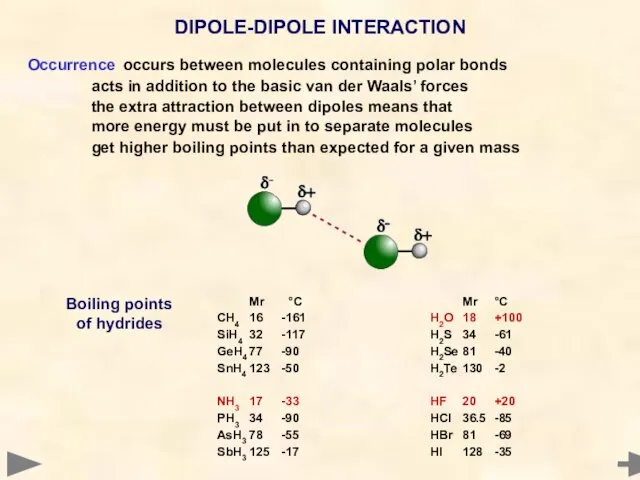
- 46. Occurrence occurs between molecules containing polar bonds acts in addition to the basic van der Waals’
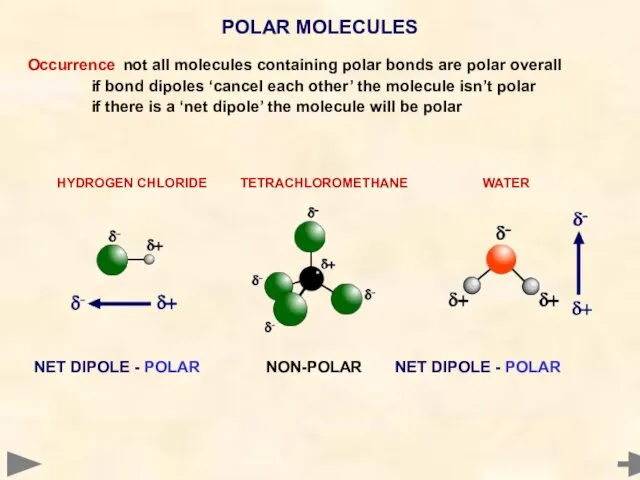
- 47. Occurrence not all molecules containing polar bonds are polar overall if bond dipoles ‘cancel each other’
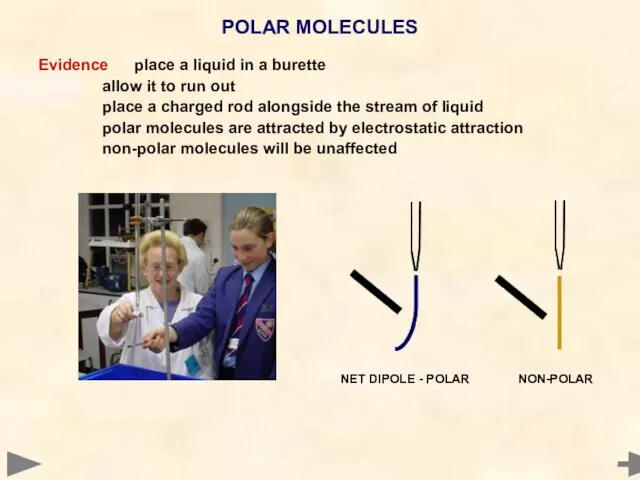
- 48. Evidence place a liquid in a burette allow it to run out place a charged rod
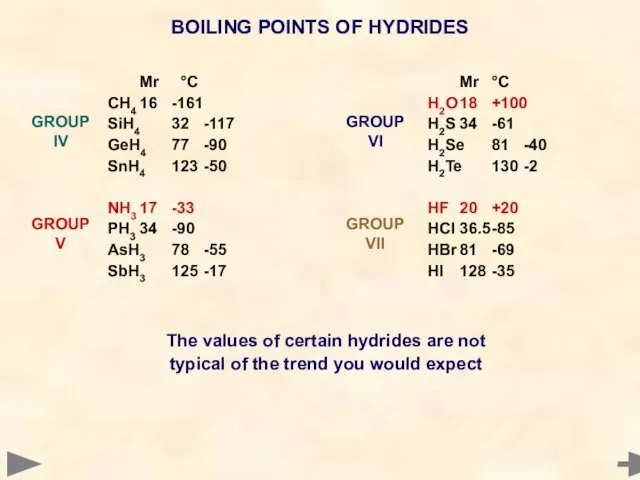
- 49. BOILING POINTS OF HYDRIDES Mr °C CH4 16 -161 SiH4 32 -117 GeH4 77 -90 SnH4
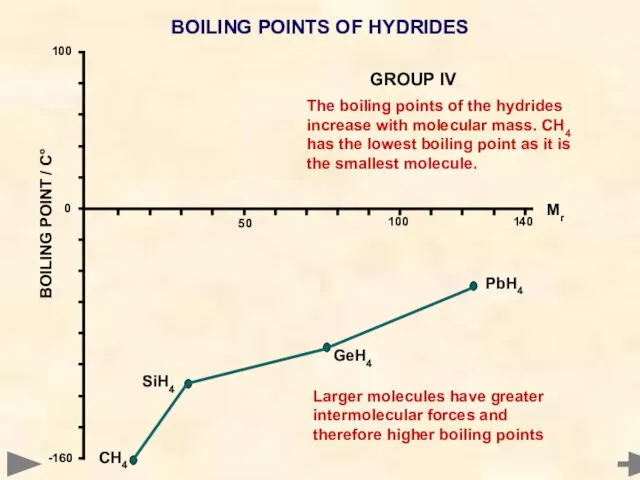
- 50. BOILING POINTS OF HYDRIDES The boiling points of the hydrides increase with molecular mass. CH4 has
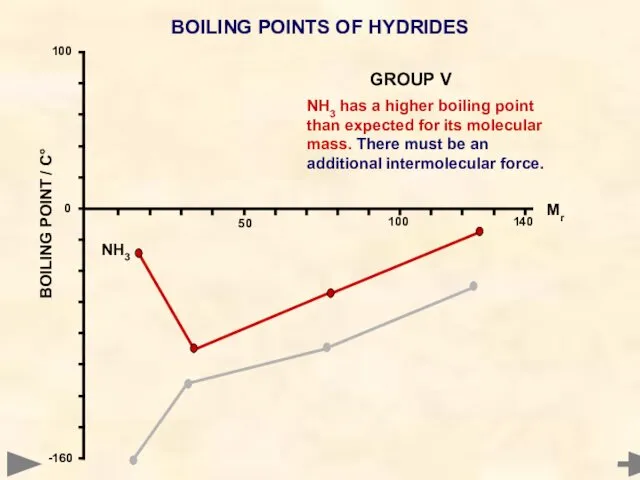
- 51. BOILING POINTS OF HYDRIDES NH3 has a higher boiling point than expected for its molecular mass.
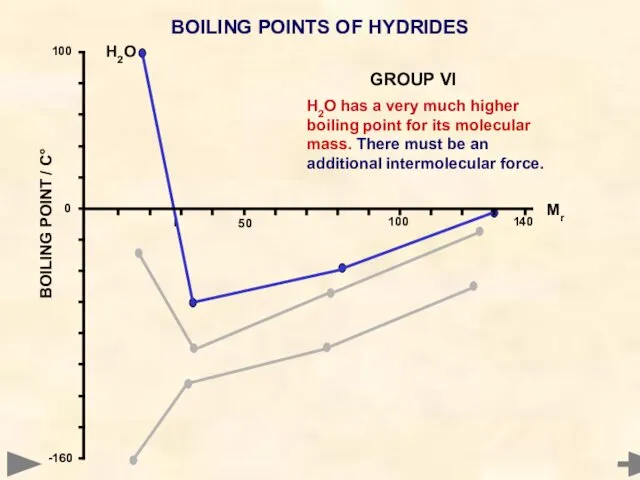
- 52. BOILING POINTS OF HYDRIDES H2O has a very much higher boiling point for its molecular mass.
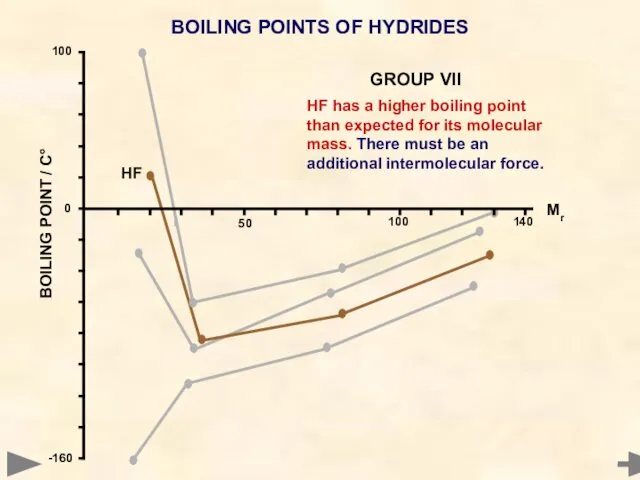
- 53. BOILING POINTS OF HYDRIDES HF has a higher boiling point than expected for its molecular mass.
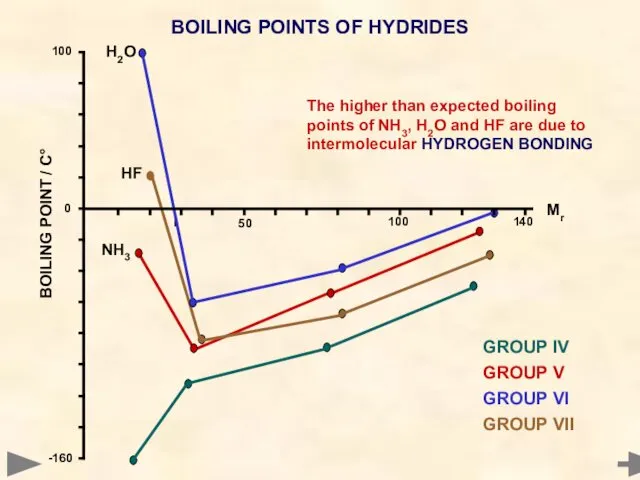
- 54. BOILING POINTS OF HYDRIDES GROUP IV GROUP V GROUP VI GROUP VII H2O HF NH3 The
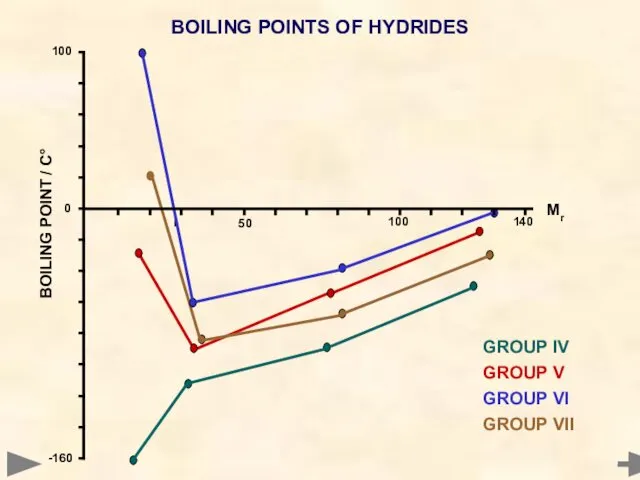
- 55. BOILING POINTS OF HYDRIDES GROUP IV GROUP V GROUP VI GROUP VII
- 56. an extension of dipole-dipole interaction gives rise to even higher boiling points bonds between H and
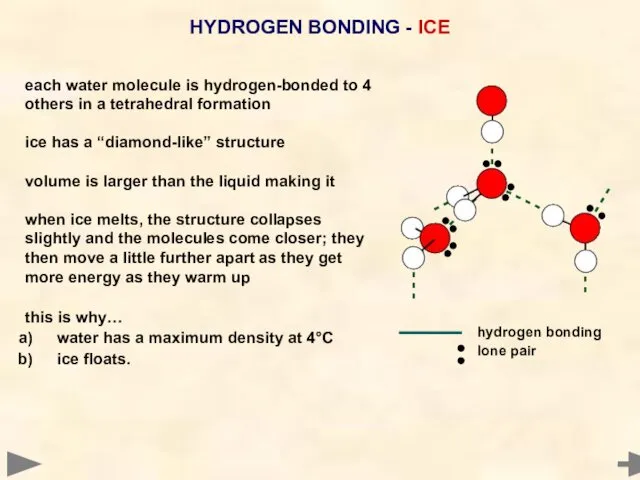
- 57. HYDROGEN BONDING - ICE each water molecule is hydrogen-bonded to 4 others in a tetrahedral formation
- 58. HYDROGEN BONDING - ICE hydrogen bonding
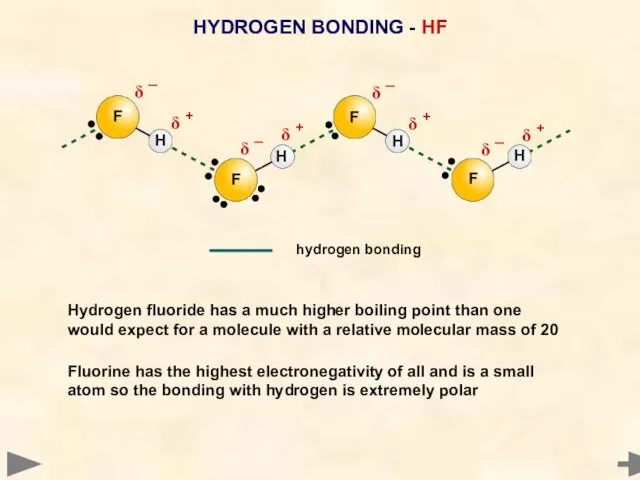
- 59. HYDROGEN BONDING - HF Hydrogen fluoride has a much higher boiling point than one would expect
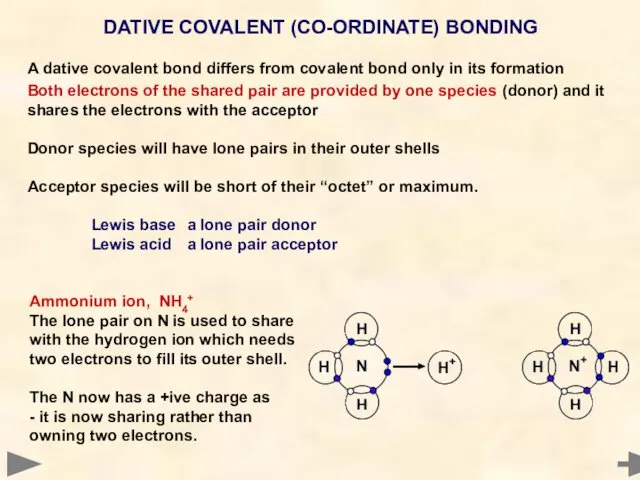
- 60. A dative covalent bond differs from covalent bond only in its formation Both electrons of the
- 61. Boron trifluoride-ammonia NH3BF3 Boron has an incomplete shell in BF3 and can accept a share of
- 62. MOLECULAR SOLIDS
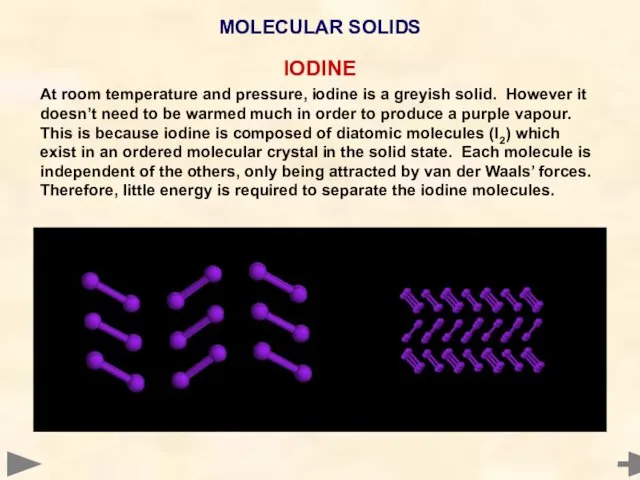
- 63. IODINE At room temperature and pressure, iodine is a greyish solid. However it doesn’t need to
- 64. COVALENT NETWORKS GIANT MOLECULES MACROMOLECULES They all mean the same!
- 65. DIAMOND, GRAPHITE and SILICA Many atoms joined together in a regular array by a large number
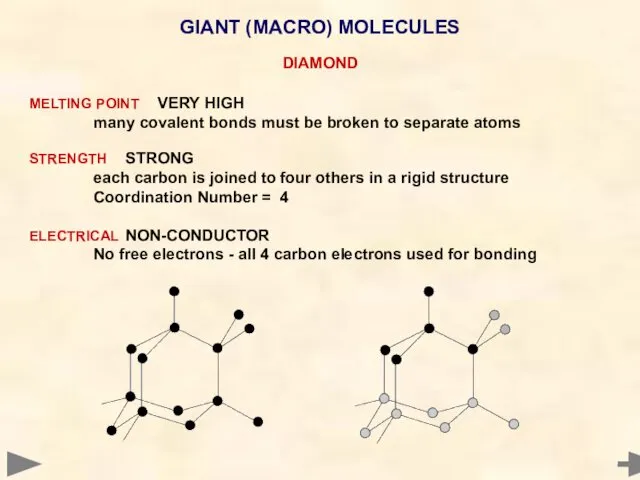
- 66. GIANT (MACRO) MOLECULES DIAMOND MELTING POINT VERY HIGH many covalent bonds must be broken to separate
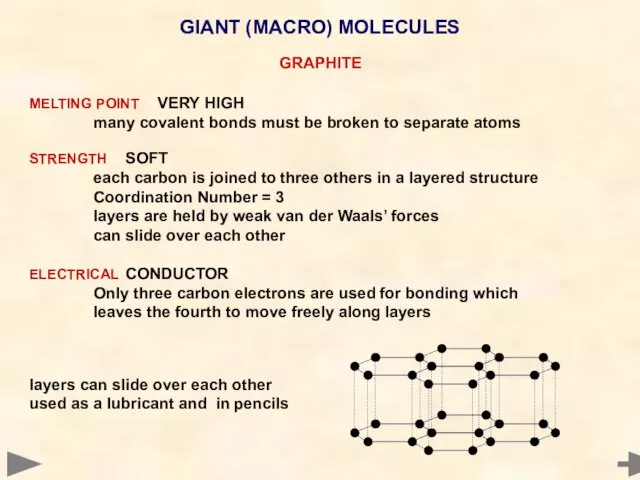
- 67. GIANT (MACRO) MOLECULES GRAPHITE MELTING POINT VERY HIGH many covalent bonds must be broken to separate
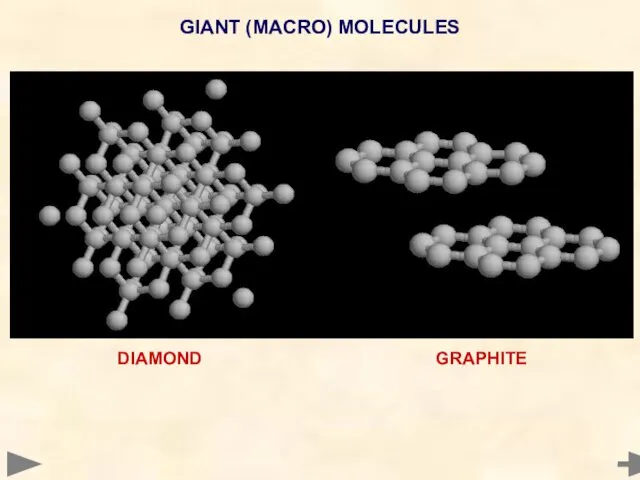
- 68. GIANT (MACRO) MOLECULES DIAMOND GRAPHITE
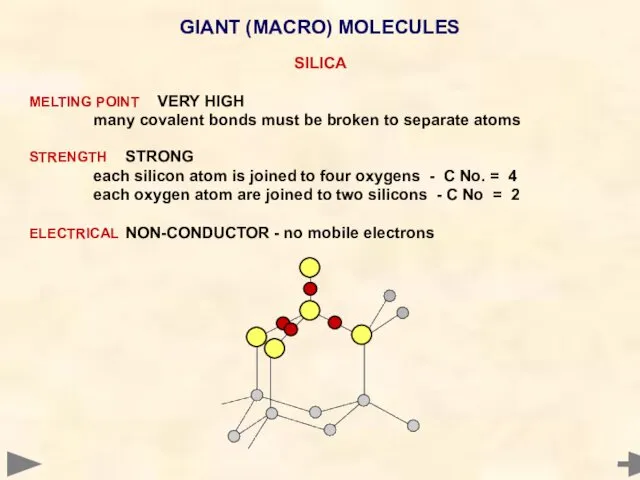
- 69. GIANT (MACRO) MOLECULES SILICA MELTING POINT VERY HIGH many covalent bonds must be broken to separate
- 70. METALLIC BONDING
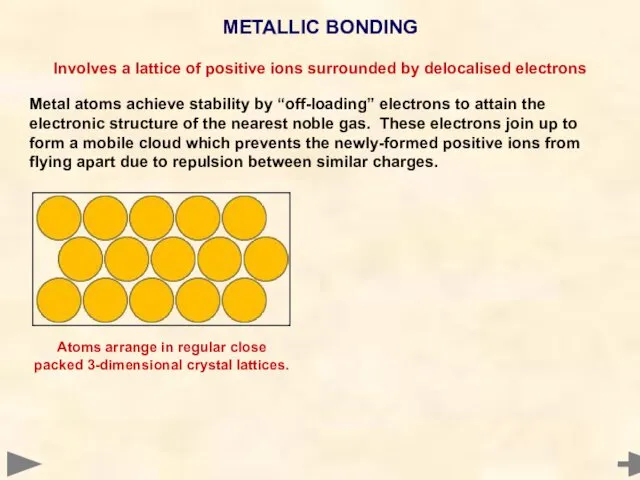
- 71. METALLIC BONDING Involves a lattice of positive ions surrounded by delocalised electrons Metal atoms achieve stability
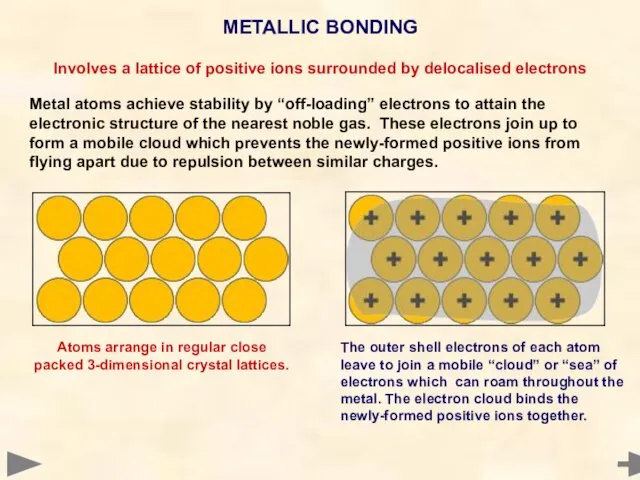
- 72. METALLIC BONDING Involves a lattice of positive ions surrounded by delocalised electrons Metal atoms achieve stability
- 73. METALLIC BONDING Involves a lattice of positive ions surrounded by delocalised electrons Metal atoms achieve stability
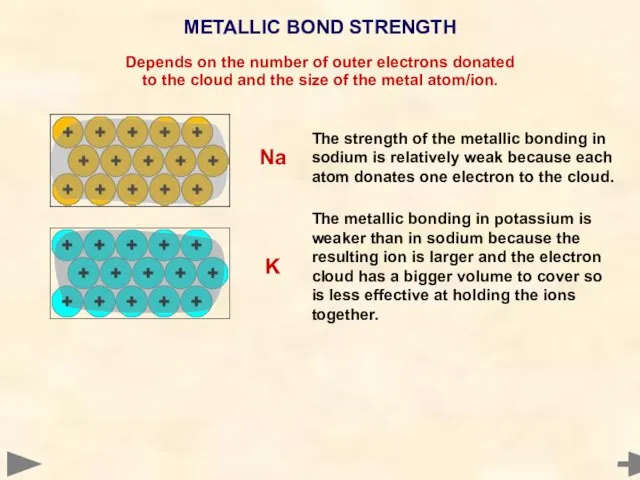
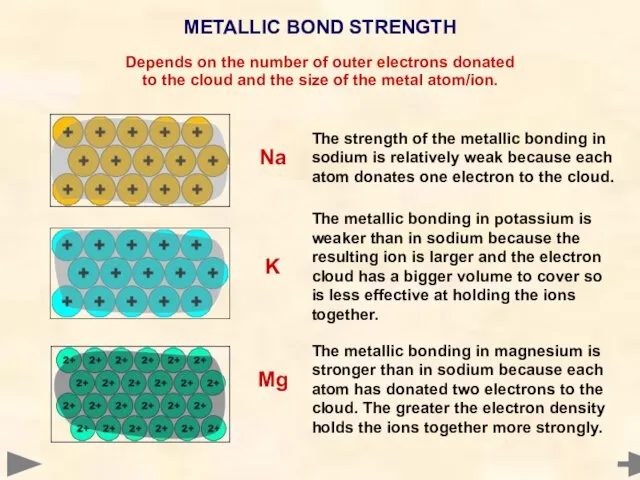
- 74. METALLIC BOND STRENGTH Depends on the number of outer electrons donated to the cloud and the
- 75. METALLIC BOND STRENGTH Depends on the number of outer electrons donated to the cloud and the
- 76. METALLIC BOND STRENGTH Depends on the number of outer electrons donated to the cloud and the
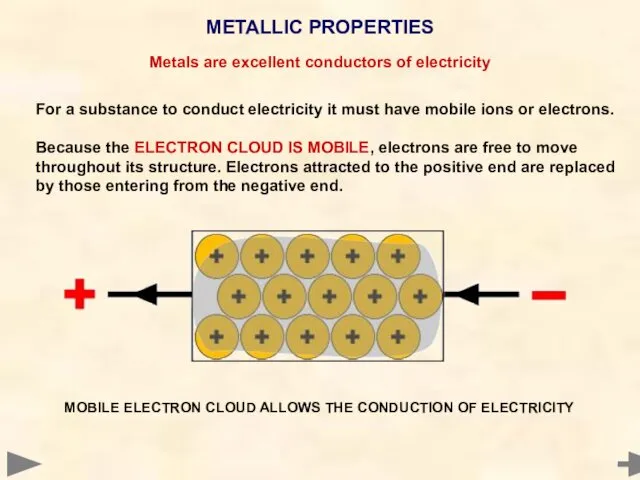
- 77. METALLIC PROPERTIES MOBILE ELECTRON CLOUD ALLOWS THE CONDUCTION OF ELECTRICITY For a substance to conduct electricity
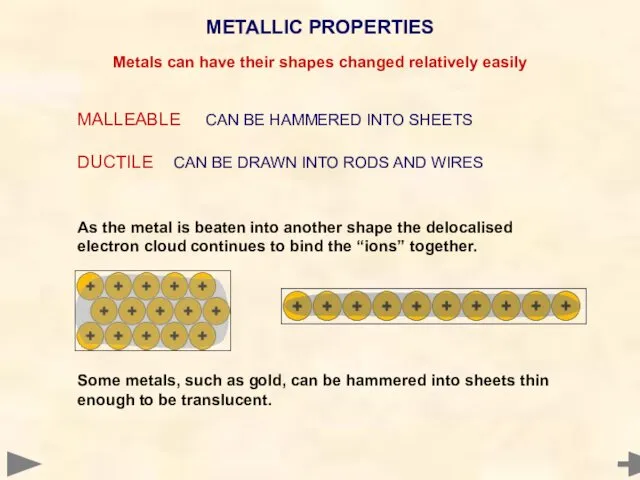
- 78. MALLEABLE CAN BE HAMMERED INTO SHEETS DUCTILE CAN BE DRAWN INTO RODS AND WIRES As the
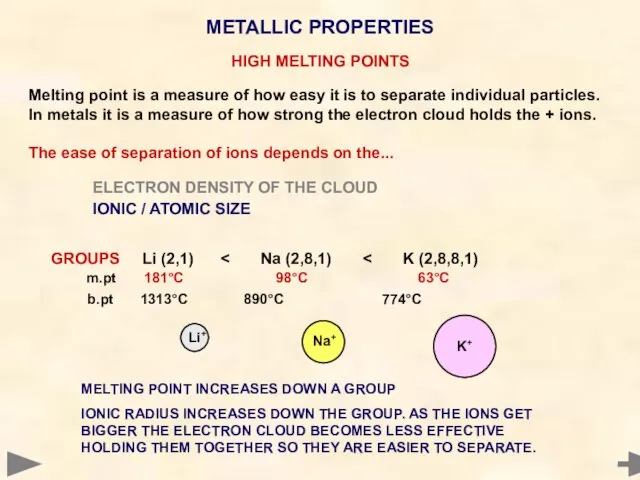
- 79. HIGH MELTING POINTS Melting point is a measure of how easy it is to separate individual
- 80. HIGH MELTING POINTS Melting point is a measure of how easy it is to separate individual
- 81. REVISION CHECK What should you be able to do? Recall the different types of physical and
- 82. You need to go over the relevant topic(s) again Click on the button to return to
- 83. WELL DONE! Try some past paper questions
- 85. Скачать презентацию























 Дослідження розчинності речовин природознавство
Дослідження розчинності речовин природознавство Францій БІОЛОГІЧНА РОЛЬ
Францій БІОЛОГІЧНА РОЛЬ  Электрохимические анализаторы медицинского назначения
Электрохимические анализаторы медицинского назначения Природні джерела вуглеводнів і їх переробка
Природні джерела вуглеводнів і їх переробка Составы и температуры плавления смесей
Составы и температуры плавления смесей Химическая коррозия
Химическая коррозия Валентность. Порядок действий при составлении химической формулы
Валентность. Порядок действий при составлении химической формулы Химия өнеркәсібіндегі энергетикалық ресурстар
Химия өнеркәсібіндегі энергетикалық ресурстар Что такое полупроводники
Что такое полупроводники Направление окислительно-восстановительного процесса
Направление окислительно-восстановительного процесса Приготовление основного и рабочих растворов хлорной извести
Приготовление основного и рабочих растворов хлорной извести Презентация по Химии "Вклад Д.И. Менделеева в развитие агрохимии. Значение его вклада в современном сельском хозяйстве" - скач
Презентация по Химии "Вклад Д.И. Менделеева в развитие агрохимии. Значение его вклада в современном сельском хозяйстве" - скач Гибридизация атомных орбиталей
Гибридизация атомных орбиталей Растворение. Растворимость веществ в воде. 8 класс
Растворение. Растворимость веществ в воде. 8 класс гетероциклич
гетероциклич Железо-углеродистые сплавы
Железо-углеродистые сплавы Изомерия - презентация_
Изомерия - презентация_ Презентация по Химии "Избранные главы металлоорганической химии" - скачать смотреть
Презентация по Химии "Избранные главы металлоорганической химии" - скачать смотреть  Сшивка и контролируемая деструкция полиолефинов
Сшивка и контролируемая деструкция полиолефинов Типы химических реакций
Типы химических реакций Периодический закон и Периодическая система химических элементов
Периодический закон и Периодическая система химических элементов Оксиды азота
Оксиды азота Российские химические технологии
Российские химические технологии Хімічні властивості кислот
Хімічні властивості кислот Получение водорода с использованием технологии газификации угля и отходов угольной промышленности
Получение водорода с использованием технологии газификации угля и отходов угольной промышленности Гетероциклдік қосылыстар
Гетероциклдік қосылыстар Скорость химических реакций. Факторы, влияющие на скорость химической реакции
Скорость химических реакций. Факторы, влияющие на скорость химической реакции Состояние радионуклидов в различных фазах и методы его изучения
Состояние радионуклидов в различных фазах и методы его изучения