Содержание
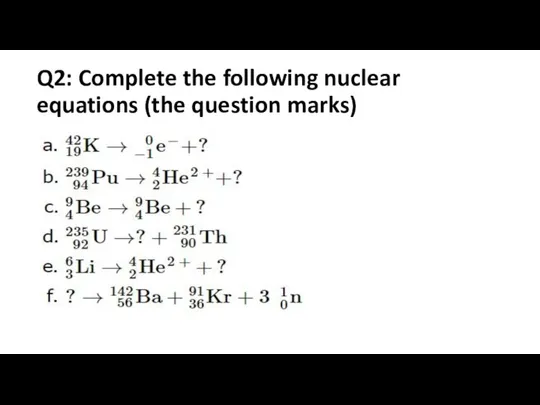
- 2. Q2: Complete the following nuclear equations (the question marks)
- 3. Pre-lesson activity: What is the atomic mass? Why we do not use the absolute atomic mass?
- 4. Theme of the lesson Atomic mass
- 5. Learning objectives Calculate relative atomic, molecular and formula masses. Explain why the atomic masses in the
- 6. Success criteria Student achieves if He/she will be able to calculate relative atomic, molecular and formula
- 7. The relative atomic mass is calculated using the equation:
- 9. Скачать презентацию





 Презентация по Химии "Закон сохранения массы вещества" - скачать смотреть
Презентация по Химии "Закон сохранения массы вещества" - скачать смотреть  Флюорит
Флюорит Презентация по Химии "Апатит" - скачать смотреть
Презентация по Химии "Апатит" - скачать смотреть  Природный газ
Природный газ Коллоидная химия
Коллоидная химия Органические производные трехвалентного фосфора
Органические производные трехвалентного фосфора Нефть и способы её переработки
Нефть и способы её переработки Сутегі. Оттегі. Сутекті алу және оның қасиеттерін зерттеу
Сутегі. Оттегі. Сутекті алу және оның қасиеттерін зерттеу Общая химия, понятия
Общая химия, понятия Сульфиды. Занятие 7
Сульфиды. Занятие 7 Типы, состав, структура, свойства РНК. (Лекция 5)
Типы, состав, структура, свойства РНК. (Лекция 5) Устойчивость дисперсных систем
Устойчивость дисперсных систем Основания. Гидроксид железа (II)
Основания. Гидроксид железа (II) Щелочные металлы
Щелочные металлы Презентация по Химии "Закон збереження маси" - скачать смотреть
Презентация по Химии "Закон збереження маси" - скачать смотреть  Аминокислоты. Белки. Пептиды
Аминокислоты. Белки. Пептиды Положение металлов в ПСХЭ Д.И. Менделеева. Общие физические свойства металлов
Положение металлов в ПСХЭ Д.И. Менделеева. Общие физические свойства металлов Презентация по Химии "Группа веществ, изолируемых из биологического материала дистилляцией («Летучие яды»)" - скачать смотрет
Презентация по Химии "Группа веществ, изолируемых из биологического материала дистилляцией («Летучие яды»)" - скачать смотрет Ցեմենտի արտադրություն
Ցեմենտի արտադրություն Альдоль-кротоновая конденсация альдегидов и кетонов
Альдоль-кротоновая конденсация альдегидов и кетонов Презентация Строение Бензола
Презентация Строение Бензола Химические элементы в нашем организме. Автор: Георгиева Татьяна Григорьевна Учитель химии и экологии МОУ «Благ
Химические элементы в нашем организме. Автор: Георгиева Татьяна Григорьевна Учитель химии и экологии МОУ «Благ Получение азотной кислоты
Получение азотной кислоты Оксиди, їх склад, назви.
Оксиди, їх склад, назви.  Программа HyperChem
Программа HyperChem Органикалық химия
Органикалық химия Обобщение сведений о важнейших классах неорганических соединений
Обобщение сведений о важнейших классах неорганических соединений Мило та його склад
Мило та його склад