Содержание
- 2. Your homework -3 use a more appropriate number format, e.g. 1,000,000 = mln. Please provide the
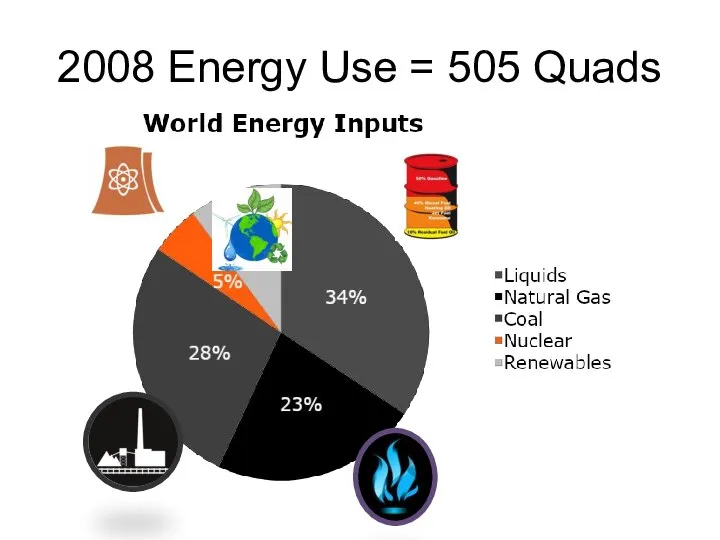
- 3. 2008 Energy Use = 505 Quads
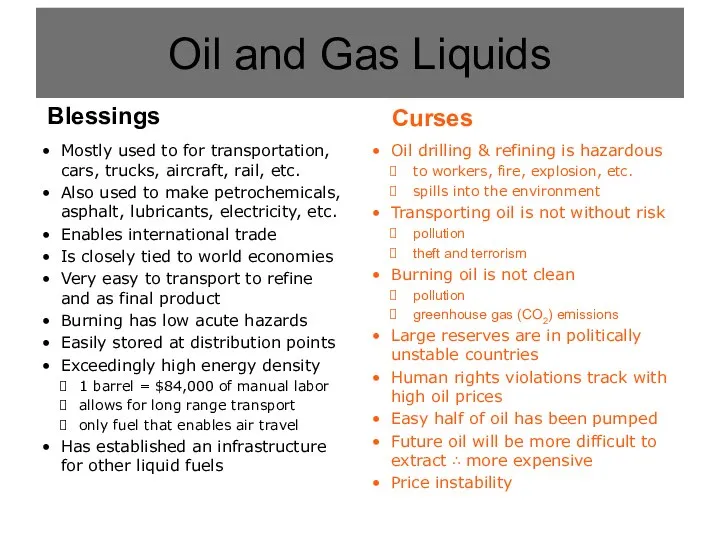
- 4. Oil and Gas Liquids
- 5. Oil drilling & refining is hazardous to workers, fire, explosion, etc. spills into the environment Transporting
- 6. Mostly used to for transportation, cars, trucks, aircraft, rail, etc. Also used to make petrochemicals, asphalt,
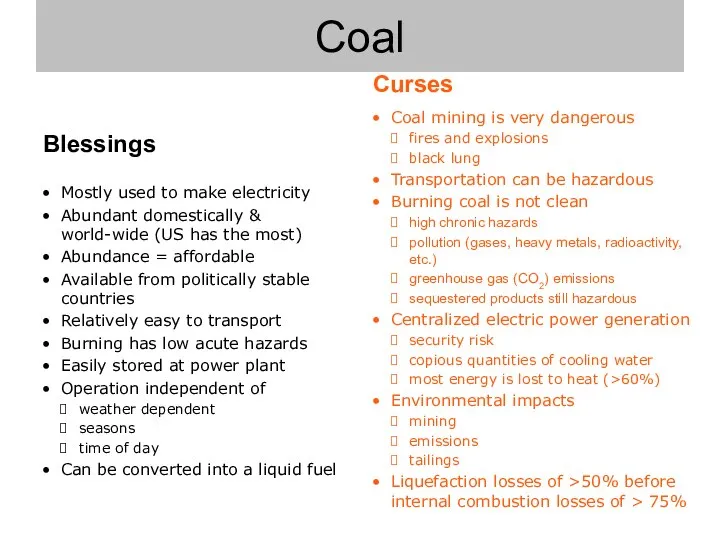
- 7. Coal
- 8. Coal mining is very dangerous fires and explosions black lung Transportation can be hazardous Burning coal
- 9. Mostly used to make electricity Abundant domestically & world-wide (US has the most) Abundance = affordable
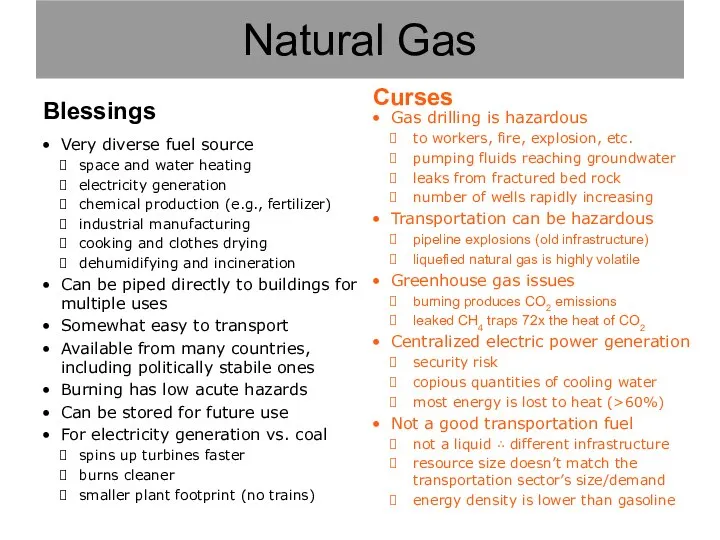
- 10. Natural Gas
- 11. Gas drilling is hazardous to workers, fire, explosion, etc. pumping fluids reaching groundwater leaks from fractured
- 12. Gas drilling is hazardous to workers, fire, explosion, etc. pumping fluids reaching groundwater leaks from fractured
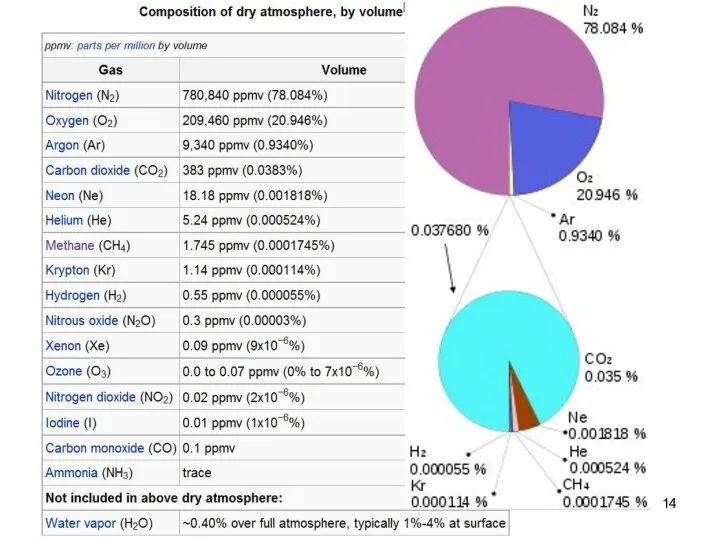
- 13. Earth atmosphere composition Lecture #3 - Energy Resources: Carbon Cycle
- 14. Lecture #3 - Energy Resources: Carbon Cycle
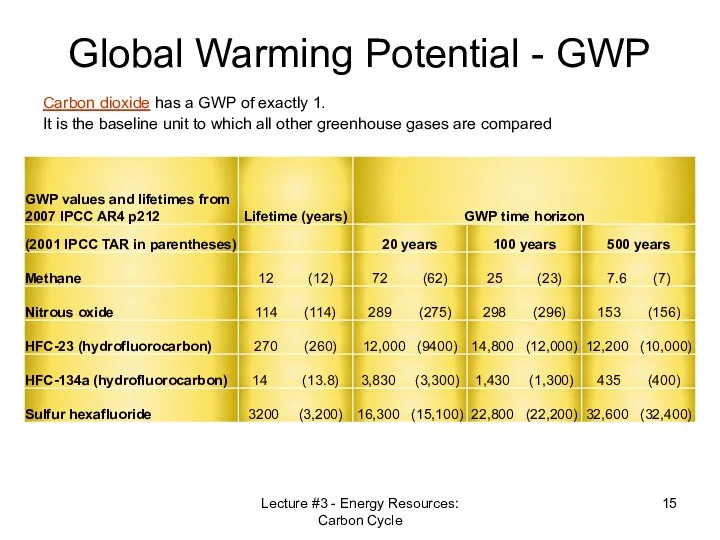
- 15. Global Warming Potential - GWP Lecture #3 - Energy Resources: Carbon Cycle Carbon dioxide has a
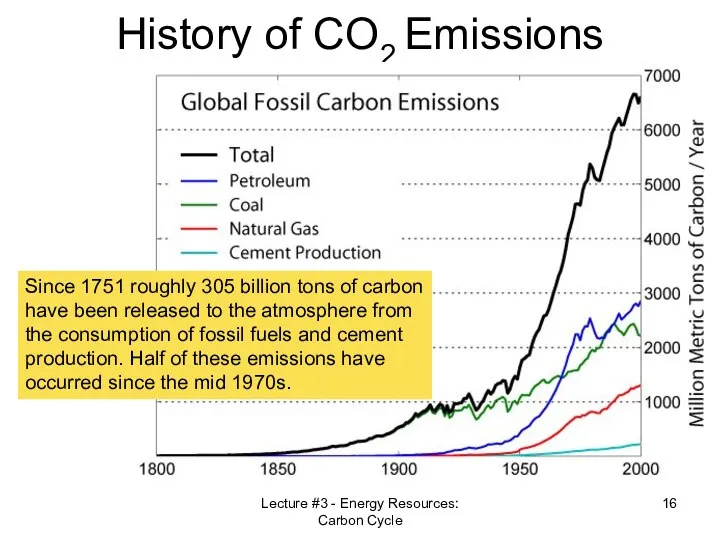
- 16. Lecture #3 - Energy Resources: Carbon Cycle History of CO2 Emissions Since 1751 roughly 305 billion
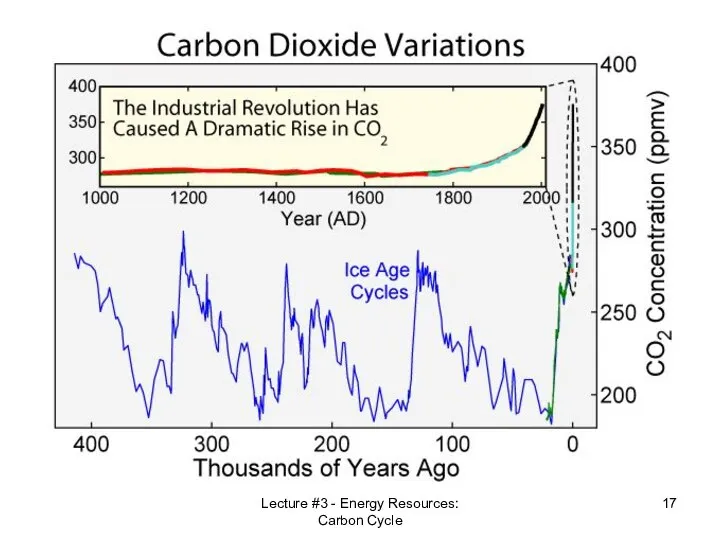
- 17. Lecture #3 - Energy Resources: Carbon Cycle
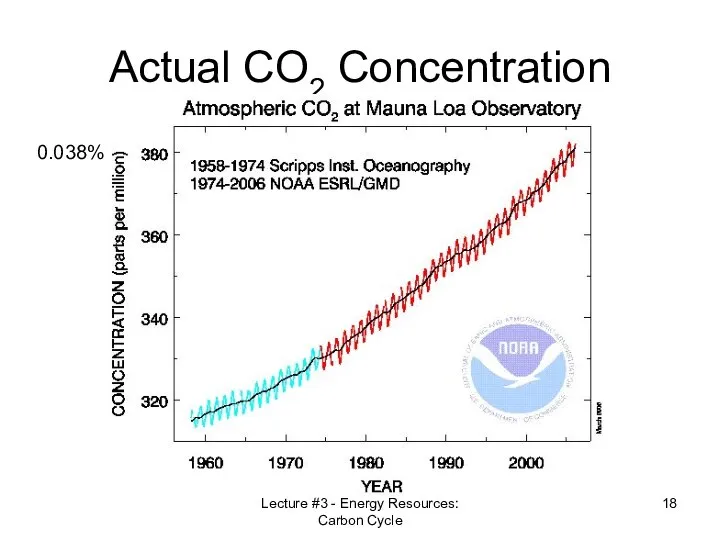
- 18. Lecture #3 - Energy Resources: Carbon Cycle Actual CO2 Concentration 0.038%
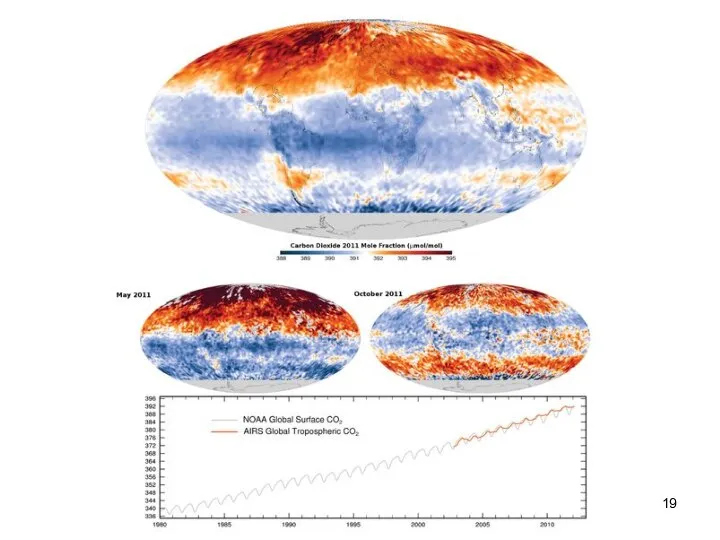
- 19. Lecture #3 - Energy Resources: Carbon Cycle
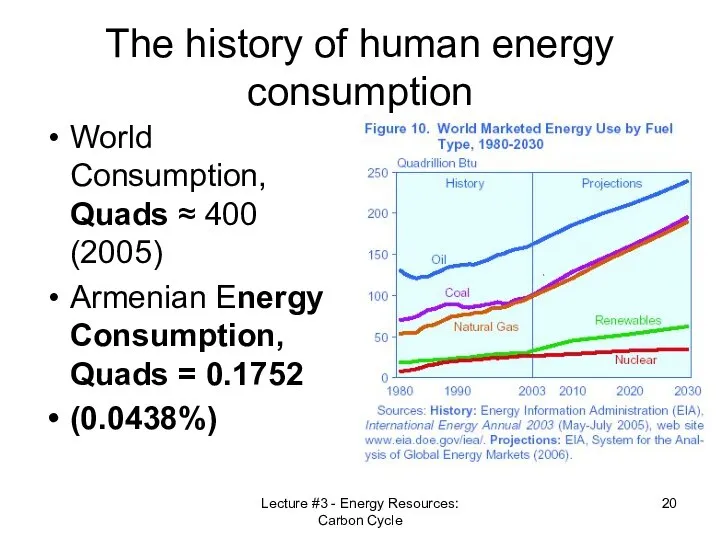
- 20. Lecture #3 - Energy Resources: Carbon Cycle The history of human energy consumption World Consumption, Quads
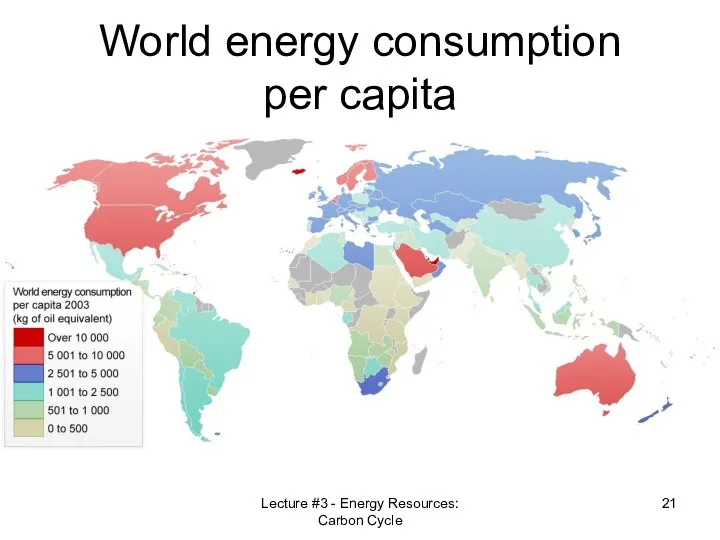
- 21. World energy consumption per capita Lecture #3 - Energy Resources: Carbon Cycle
- 22. Comparison In 2008 energy use per person was in the USA 4.1 fold, EU 1.9 fold
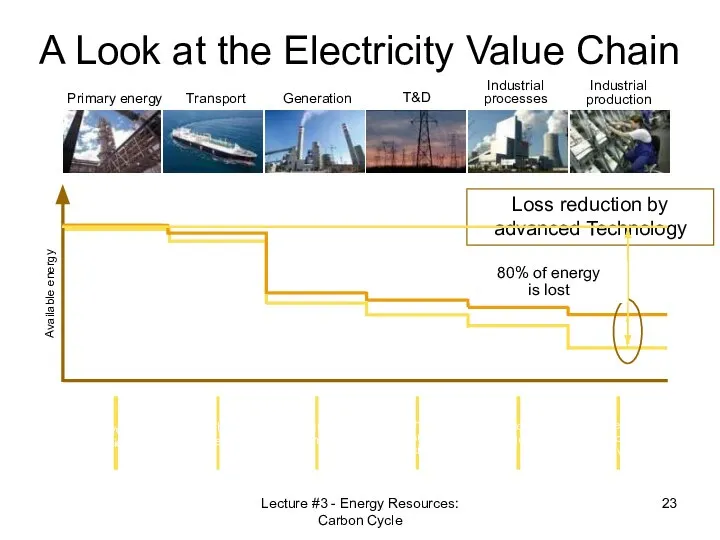
- 23. Primary energy Transport Generation T&D Industrial processes Industrial production Available energy A Look at the Electricity
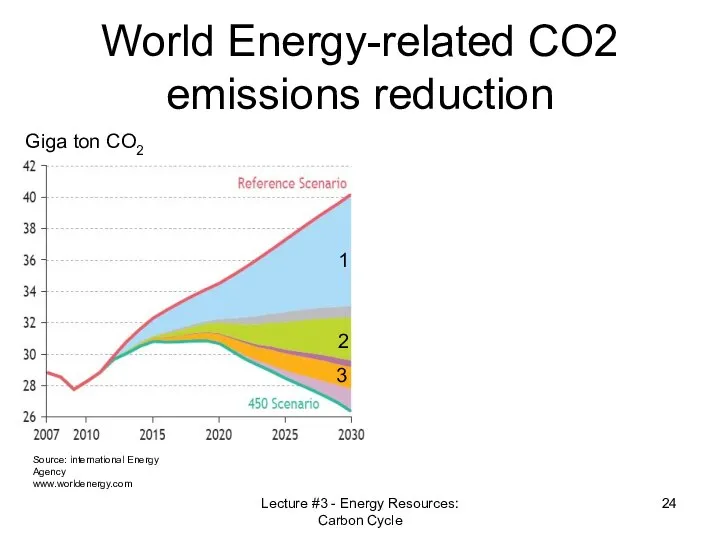
- 24. World Energy-related CO2 emissions reduction Source: international Energy Agency www.worldenergy.com Giga ton CO2 1 2 3
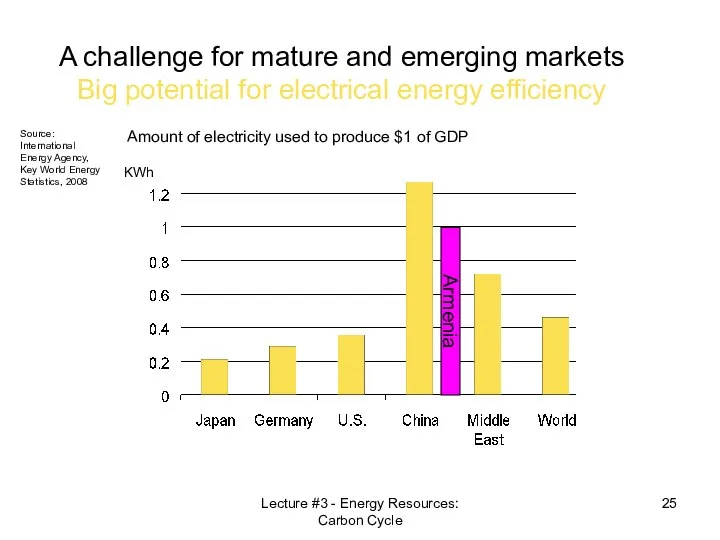
- 25. Amount of electricity used to produce $1 of GDP A challenge for mature and emerging markets
- 26. Lecture #3 - Energy Resources: Carbon Cycle 1.3 The carbon cycle and fossil fuel formation Plants
- 27. Lecture #3 - Energy Resources: Carbon Cycle 1.3 The carbon cycle and fossil fuel formation Plants
- 28. Lecture #3 - Energy Resources: Carbon Cycle Here geological times are involved
- 29. Lecture #3 - Energy Resources: Carbon Cycle 1.3 The carbon cycle and fossil fuel formation
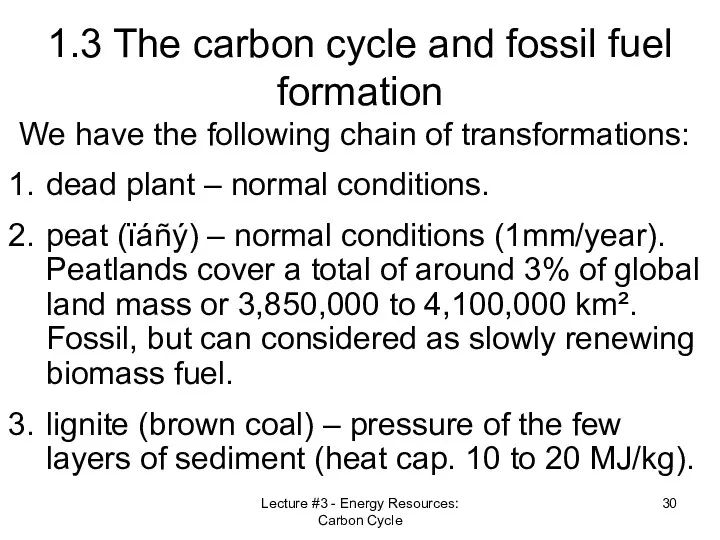
- 30. Lecture #3 - Energy Resources: Carbon Cycle 1.3 The carbon cycle and fossil fuel formation We
- 31. Lecture #3 - Energy Resources: Carbon Cycle 1.3 The carbon cycle and fossil fuel formation Now
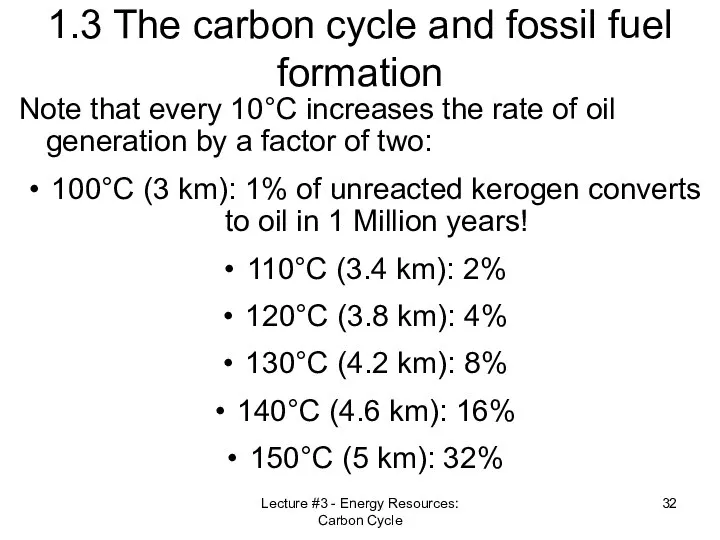
- 32. Lecture #3 - Energy Resources: Carbon Cycle 1.3 The carbon cycle and fossil fuel formation Note
- 33. Lecture #3 - Energy Resources: Carbon Cycle How much coal is needed to power a computer?
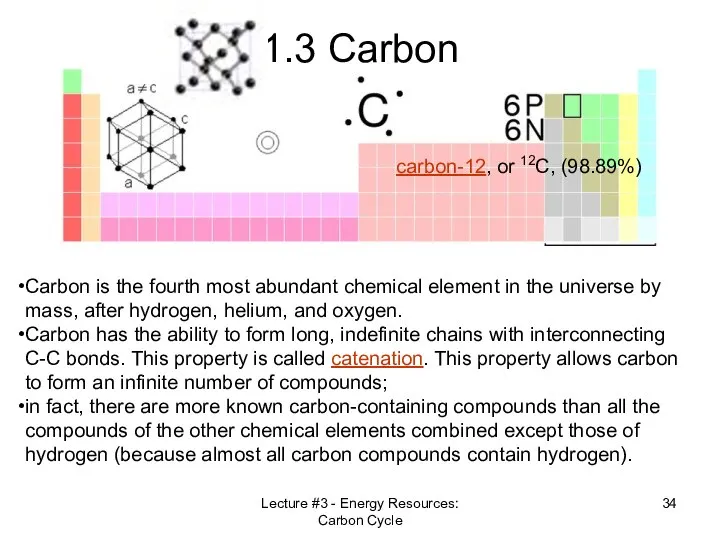
- 34. Lecture #3 - Energy Resources: Carbon Cycle 1.3 Carbon Carbon is the fourth most abundant chemical
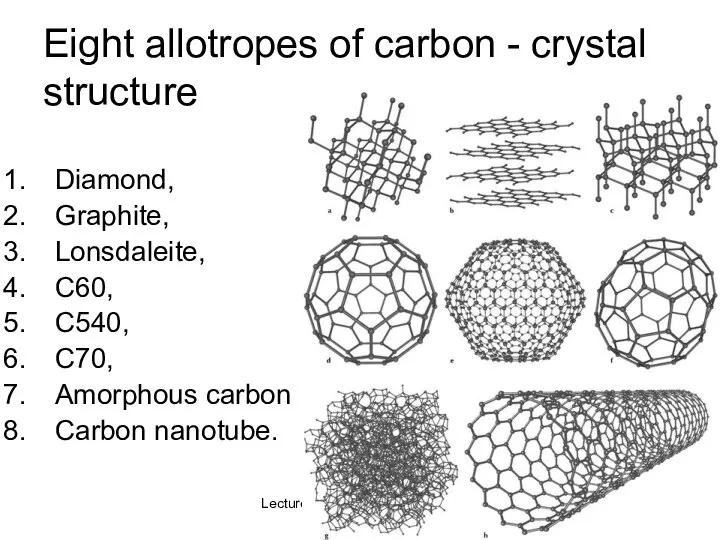
- 35. Lecture #3 - Energy Resources: Carbon Cycle Eight allotropes of carbon - crystal structure Diamond, Graphite,
- 36. Lecture #3 - Energy Resources: Carbon Cycle Hydrocarbons HydrocarbonsHydrocarbons (such as coalHydrocarbons (such as coal, petroleumHydrocarbons
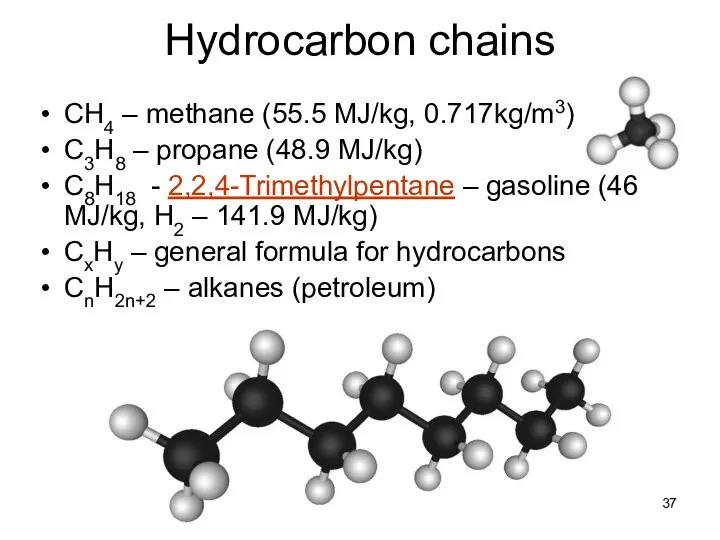
- 37. Lecture #3 - Energy Resources: Carbon Cycle Hydrocarbon chains CH4 – methane (55.5 MJ/kg, 0.717kg/m3) C3H8
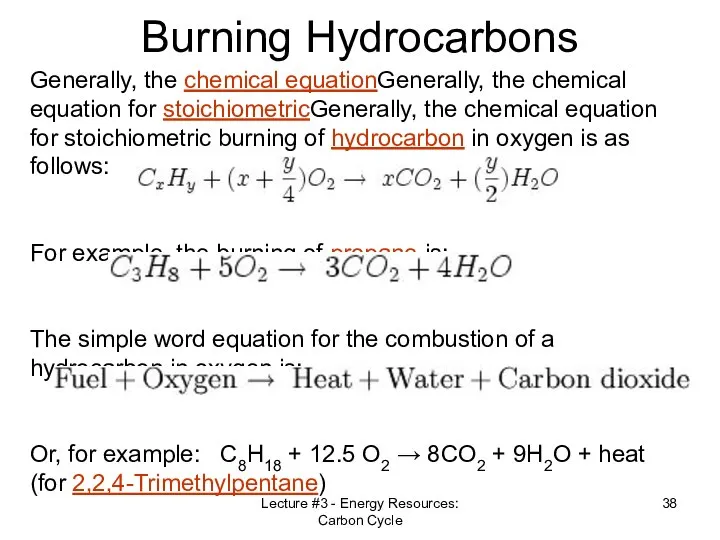
- 38. Lecture #3 - Energy Resources: Carbon Cycle Burning Hydrocarbons Generally, the chemical equationGenerally, the chemical equation
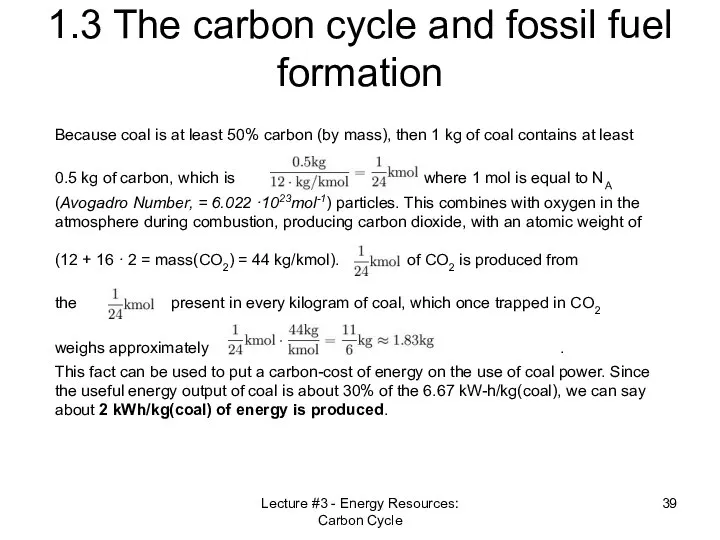
- 39. Lecture #3 - Energy Resources: Carbon Cycle 1.3 The carbon cycle and fossil fuel formation Because
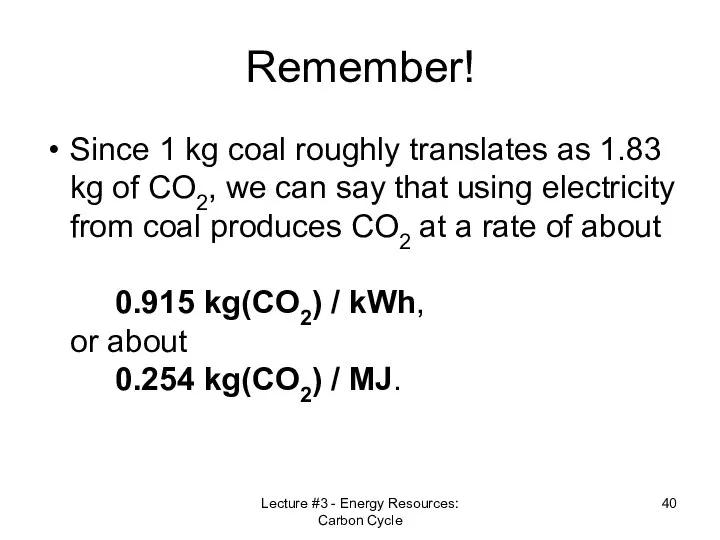
- 40. Remember! Since 1 kg coal roughly translates as 1.83 kg of CO2, we can say that
- 41. Lecture #3 - Energy Resources: Carbon Cycle Shale (ûñóù³ñ) Oil shale is a general term applied
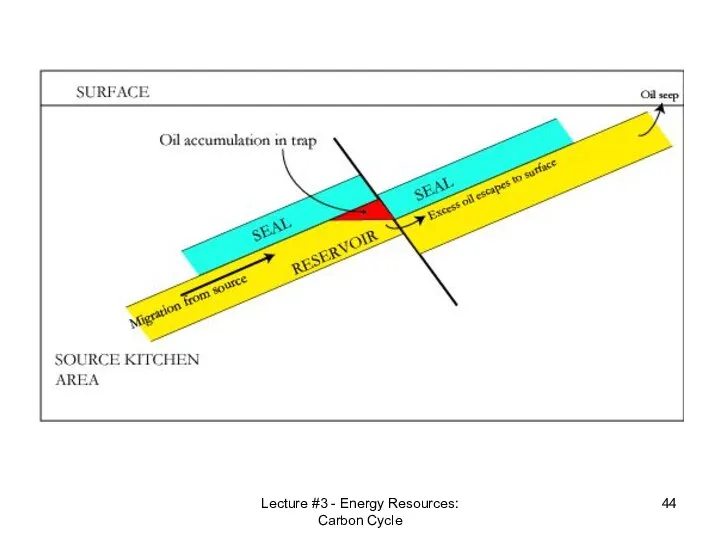
- 42. Lecture #3 - Energy Resources: Carbon Cycle Reservoir Rock An oil reservoir, petroleum system or petroleum
- 43. Lecture #3 - Energy Resources: Carbon Cycle
- 44. Lecture #3 - Energy Resources: Carbon Cycle
- 45. Lecture #3 - Energy Resources: Carbon Cycle
- 46. Lecture #3 - Energy Resources: Carbon Cycle 1.3 Economy of extraction Porosity = Volume of Void
- 47. Lecture #3 - Energy Resources: Carbon Cycle
- 48. Liquid fuel volume units The standard barrel of crude oil or other petroleum product (abbreviated bbl)
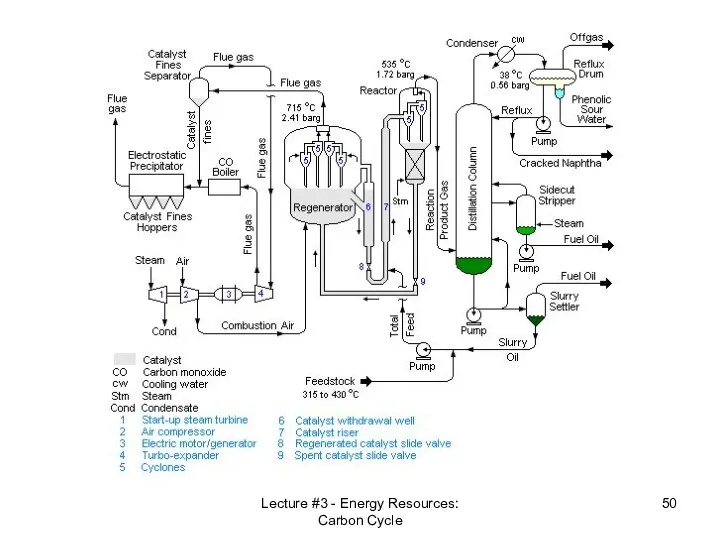
- 49. Oil extraction – gulf of Mexico Lecture #3 - Energy Resources: Carbon Cycle Oil refinery -
- 50. Lecture #3 - Energy Resources: Carbon Cycle
- 51. Oil soaked porous rock. Sample comes from offshore fields near Sicily that are too expensive to
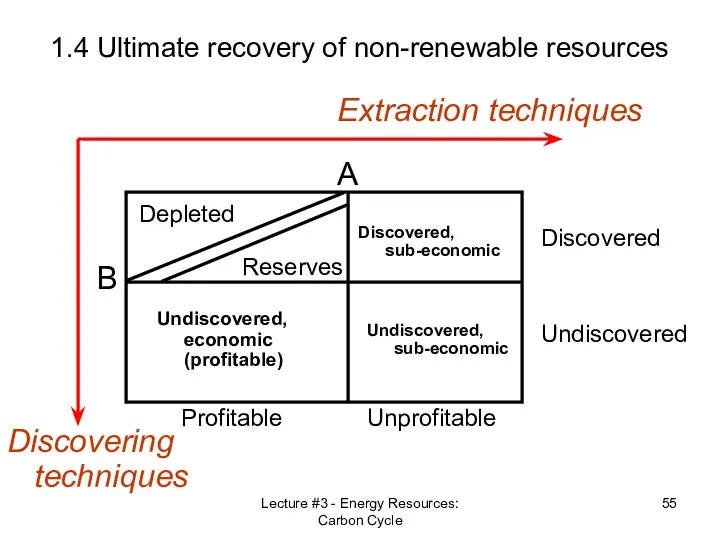
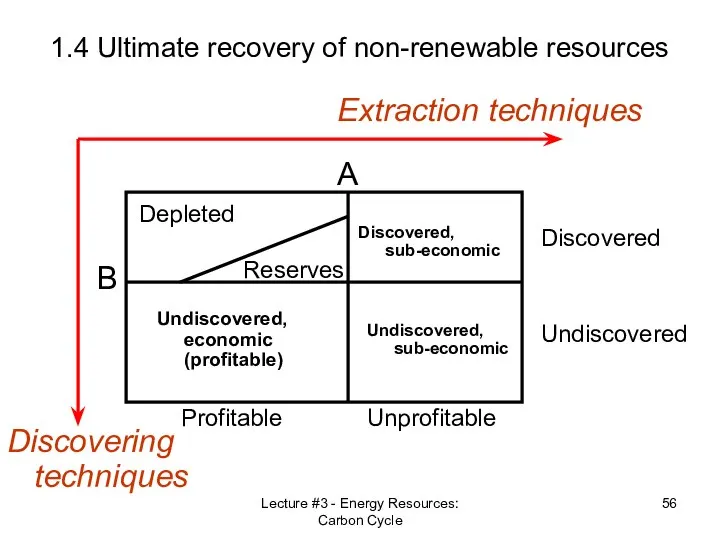
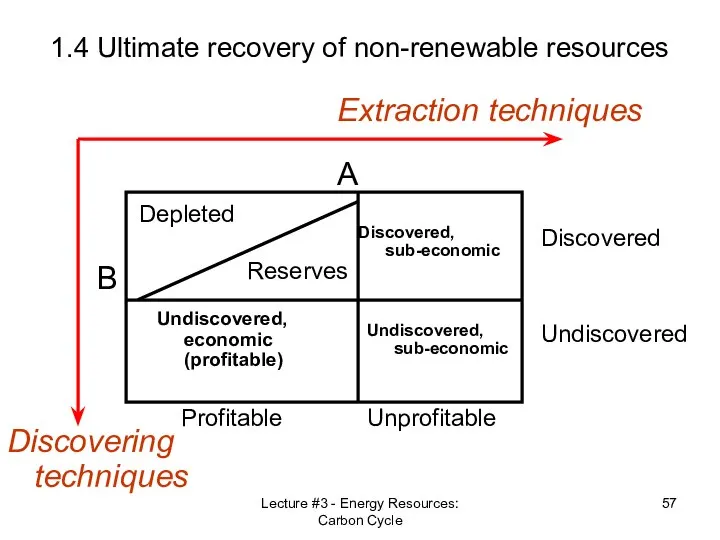
- 52. Lecture #3 - Energy Resources: Carbon Cycle 1.4 Ultimate recovery of non-renewable resources Reserves vs. Resources
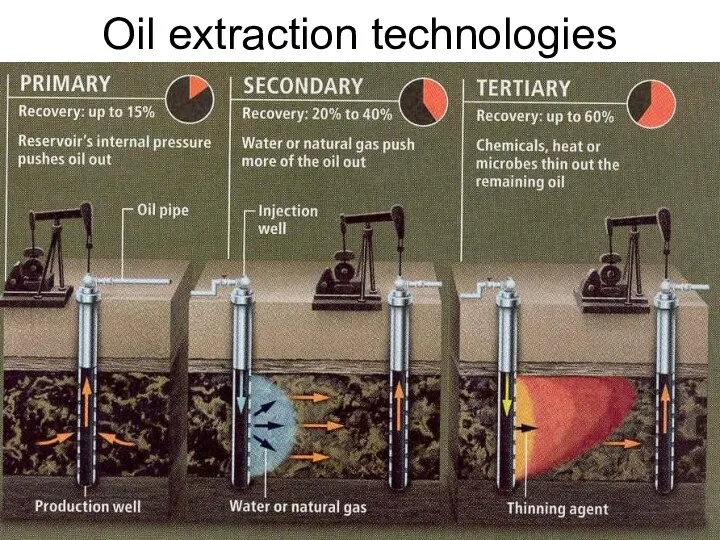
- 53. Oil extraction technologies Lecture #3 - Energy Resources: Carbon Cycle
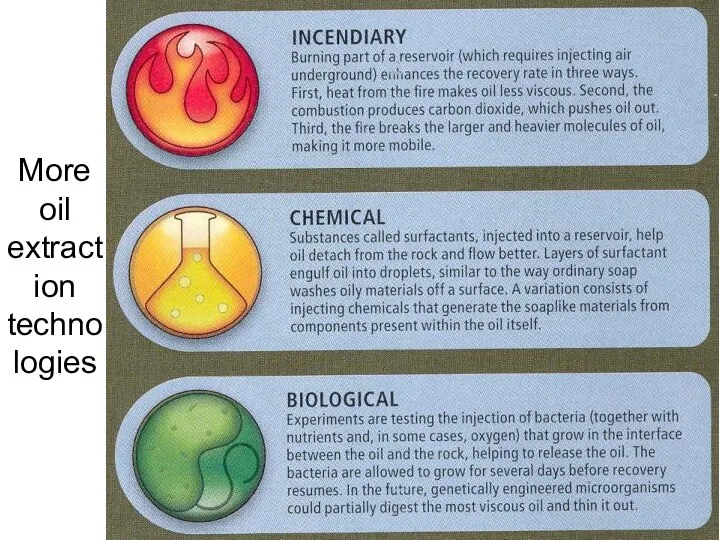
- 54. More oil extraction technologies Lecture #3 - Energy Resources: Carbon Cycle
- 55. Lecture #3 - Energy Resources: Carbon Cycle 1.4 Ultimate recovery of non-renewable resources Discovering techniques Extraction
- 56. Lecture #3 - Energy Resources: Carbon Cycle 1.4 Ultimate recovery of non-renewable resources Discovering techniques Extraction
- 57. Lecture #3 - Energy Resources: Carbon Cycle 1.4 Ultimate recovery of non-renewable resources Discovering techniques Extraction
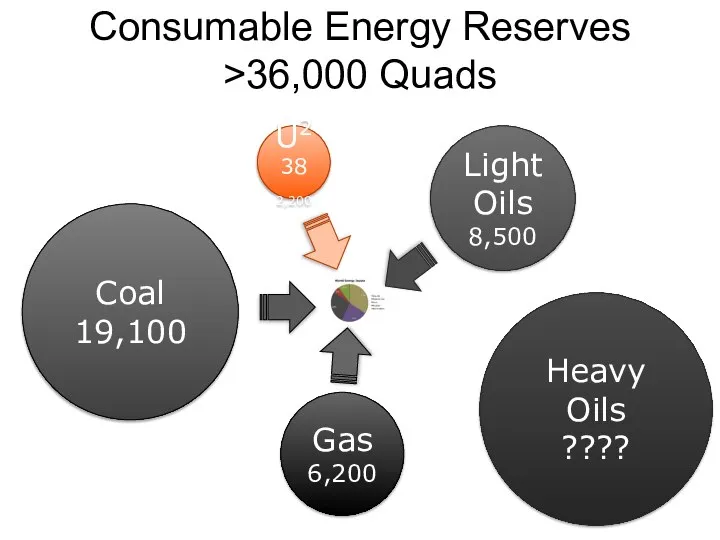
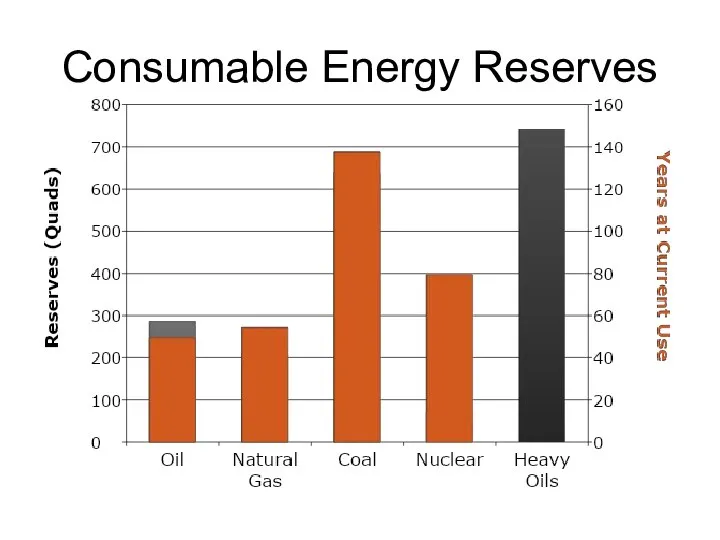
- 58. Consumable Energy Reserves >36,000 Quads Light Oils 8,500 U238 2,200 Coal 19,100 Gas 6,200 Heavy Oils
- 59. Consumable Energy Reserves
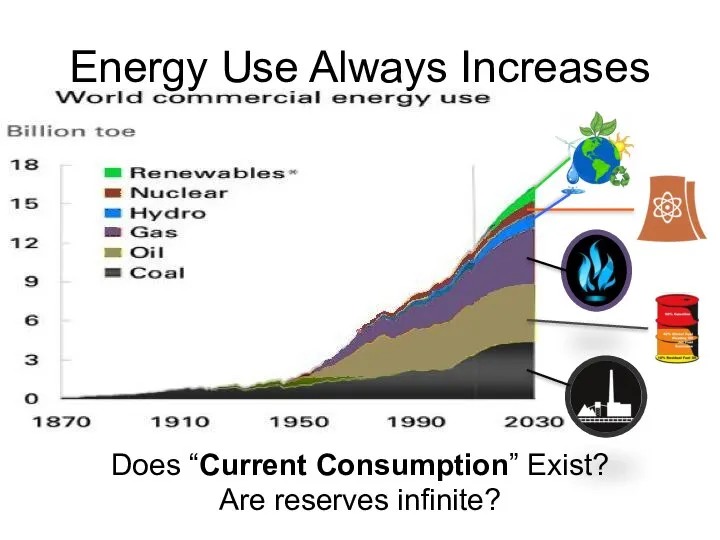
- 60. Energy Use Always Increases Does “Current Consumption” Exist? Are reserves infinite?
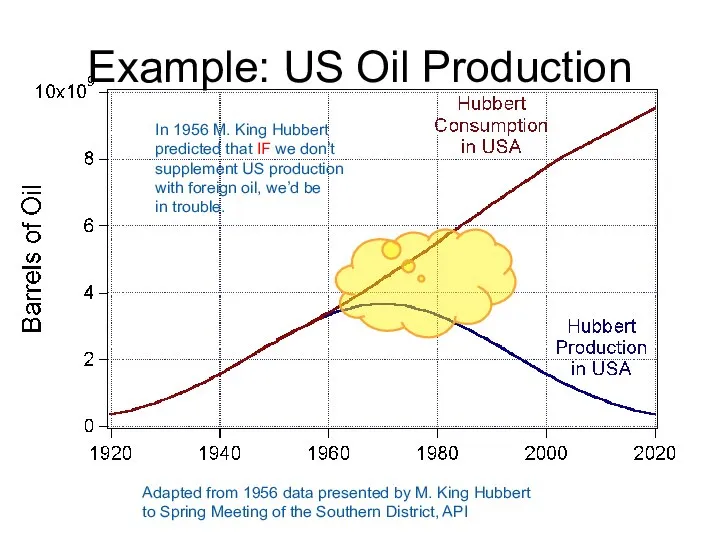
- 61. Example: US Oil Production Adapted from 1956 data presented by M. King Hubbert to Spring Meeting
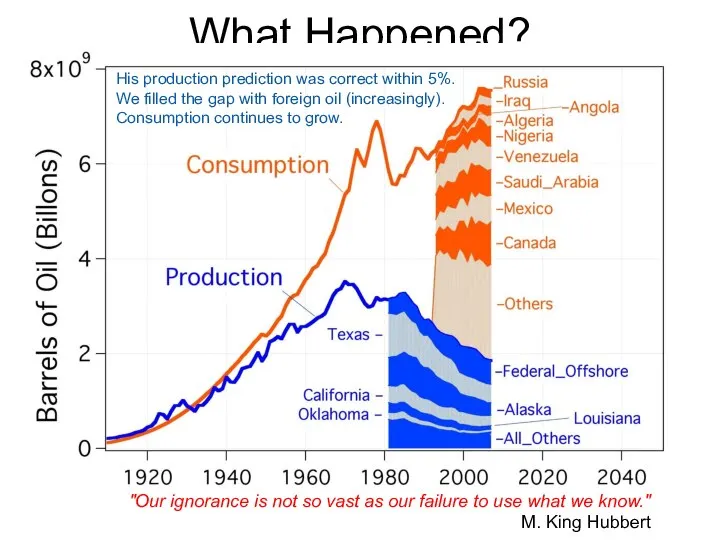
- 62. What Happened? "Our ignorance is not so vast as our failure to use what we know."
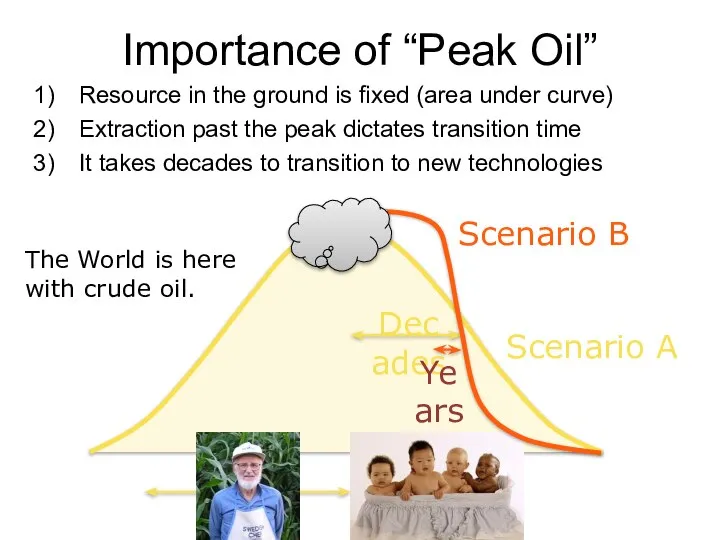
- 63. Importance of “Peak Oil” Resource in the ground is fixed (area under curve) Extraction past the
- 64. Fuels: from Hell to Heaven
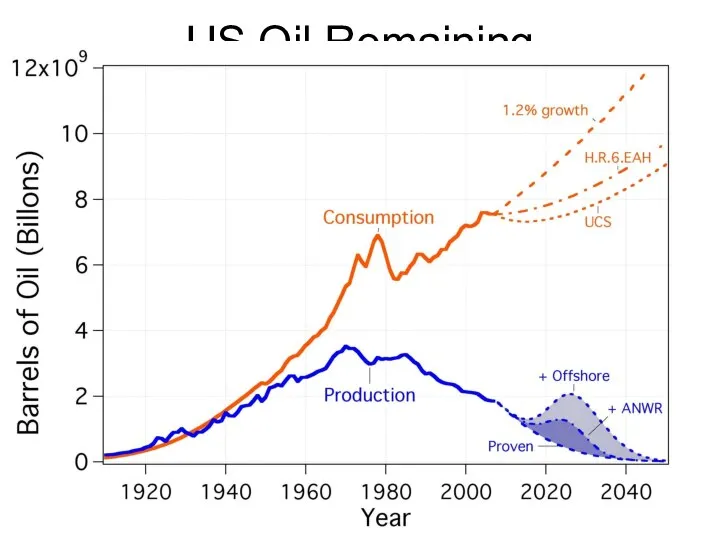
- 65. US Oil Remaining
- 66. Lecture #3 - Energy Resources: Carbon Cycle 1.5 The future of energy resources Solar Constant =
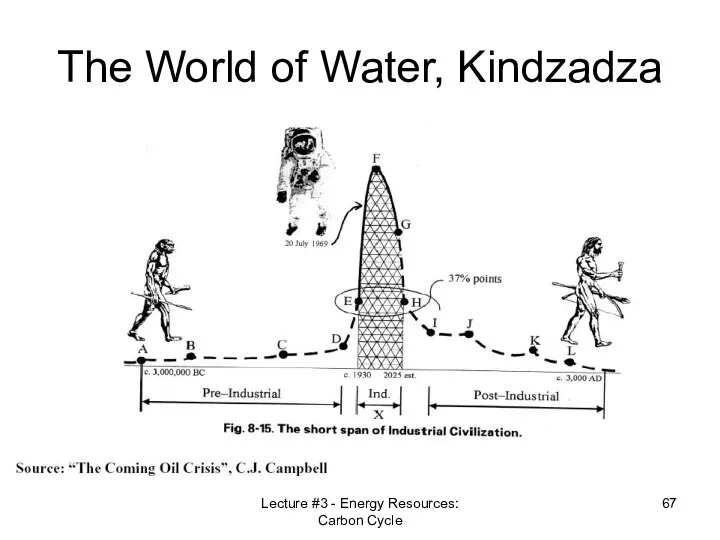
- 67. Lecture #3 - Energy Resources: Carbon Cycle The World of Water, Kindzadza
- 68. Lecture #3 - Energy Resources: Carbon Cycle
- 70. Скачать презентацию




























 Вода в меняющемся мире
Вода в меняющемся мире Уникальные свойства воды
Уникальные свойства воды Разработка проекта нормативов образования отходов и лимитов на их размещения для ОСП «УЗМК ВНЗМ»
Разработка проекта нормативов образования отходов и лимитов на их размещения для ОСП «УЗМК ВНЗМ» Ökologie und umweltverträglichkeit
Ökologie und umweltverträglichkeit Экологически допустимый вклад предприятия в загрязнение атмосферы. Структура и рекомендации по оформлению
Экологически допустимый вклад предприятия в загрязнение атмосферы. Структура и рекомендации по оформлению Биогеоценоз. Биогеоценотический уровень организации жизни. Разнообразие видов
Биогеоценоз. Биогеоценотический уровень организации жизни. Разнообразие видов Экологический проект «Мусор надо разделять, чтоб использовать опять»
Экологический проект «Мусор надо разделять, чтоб использовать опять» Кабинет экологии. МАДОУ «Детский сад №99 общеразвивающего вида»
Кабинет экологии. МАДОУ «Детский сад №99 общеразвивающего вида» Структура и свойства экосистемы
Структура и свойства экосистемы Смирновский заповедник
Смирновский заповедник Значение летнего оздоровительного лагеря для экологического образования детей в интересах устойчивого развития
Значение летнего оздоровительного лагеря для экологического образования детей в интересах устойчивого развития Презентация Кто что ест
Презентация Кто что ест  Структура экосистемы
Структура экосистемы Пластиковая бутылка – проблемы и решения
Пластиковая бутылка – проблемы и решения Методическая разработка по выполнению инновационного проекта «Цветы и время»
Методическая разработка по выполнению инновационного проекта «Цветы и время» Биологические ресурсы, их рациональное использование
Биологические ресурсы, их рациональное использование Радиационные аварии и их последствия
Радиационные аварии и их последствия Поток энергии и круговорот веществ в экосистемах
Поток энергии и круговорот веществ в экосистемах Живая нить
Живая нить Мероприятия по охране редких и исчезающих растений и животных
Мероприятия по охране редких и исчезающих растений и животных Презентация на тему Где живут белые медведи и пингвины? Урок окружающего мира 1 класс
Презентация на тему Где живут белые медведи и пингвины? Урок окружающего мира 1 класс Мониторинг радиационного загрязнения
Мониторинг радиационного загрязнения Экология пәні. Ортаның экологиялық факторлары
Экология пәні. Ортаның экологиялық факторлары Промышленная биотехнология
Промышленная биотехнология Глобальные экологические проблемы
Глобальные экологические проблемы Работа международных экологических организаций России
Работа международных экологических организаций России Сохранение и восстановление лесов
Сохранение и восстановление лесов Заказник Пижемский
Заказник Пижемский