Содержание
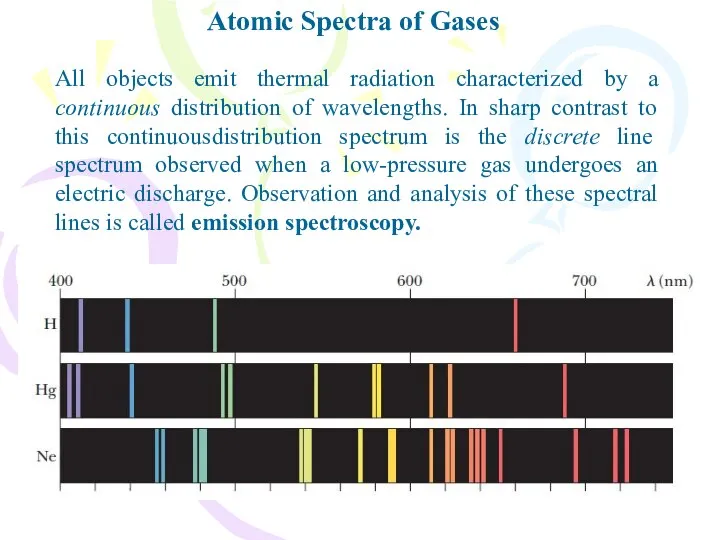
- 2. Atomic Spectra of Gases All objects emit thermal radiation characterized by a continuous distribution of wavelengths.
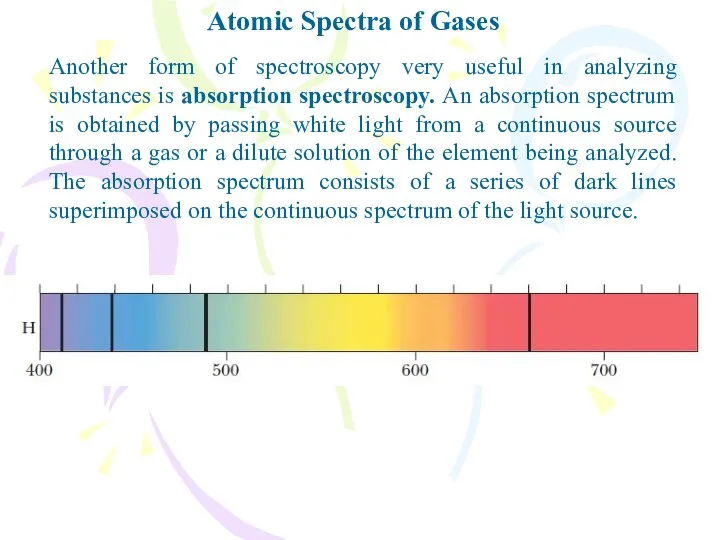
- 3. Atomic Spectra of Gases Another form of spectroscopy very useful in analyzing substances is absorption spectroscopy.
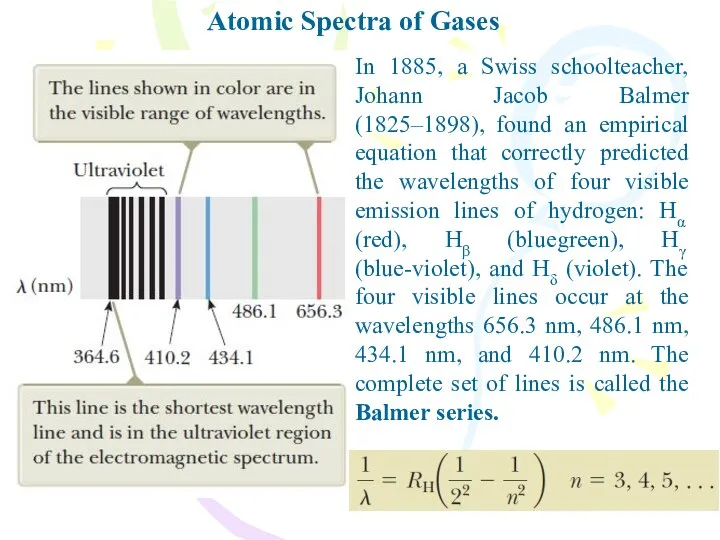
- 4. Atomic Spectra of Gases In 1885, a Swiss schoolteacher, Johann Jacob Balmer (1825–1898), found an empirical
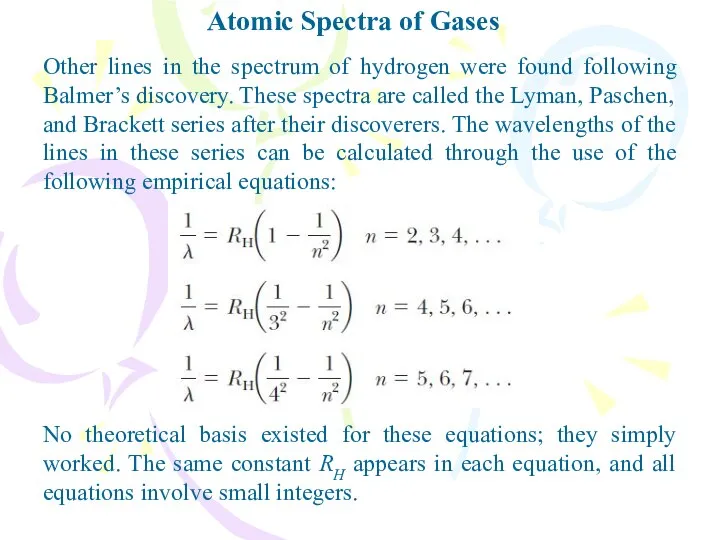
- 5. Atomic Spectra of Gases Other lines in the spectrum of hydrogen were found following Balmer’s discovery.
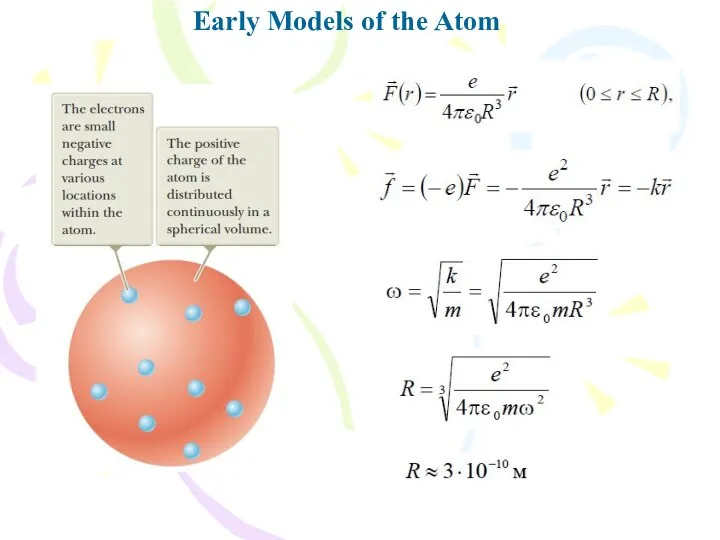
- 6. Early Models of the Atom
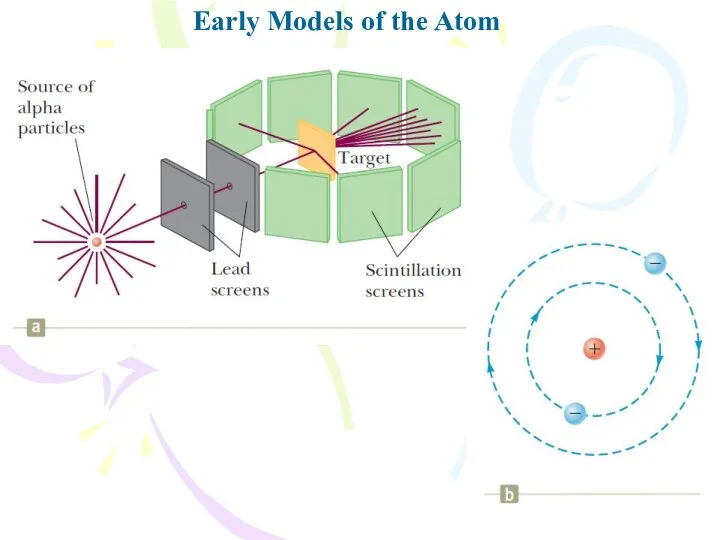
- 7. Early Models of the Atom
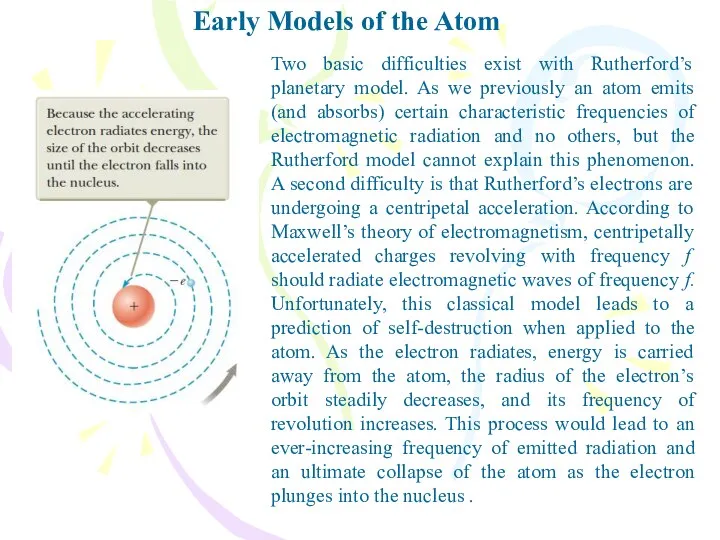
- 8. Early Models of the Atom Two basic difficulties exist with Rutherford’s planetary model. As we previously
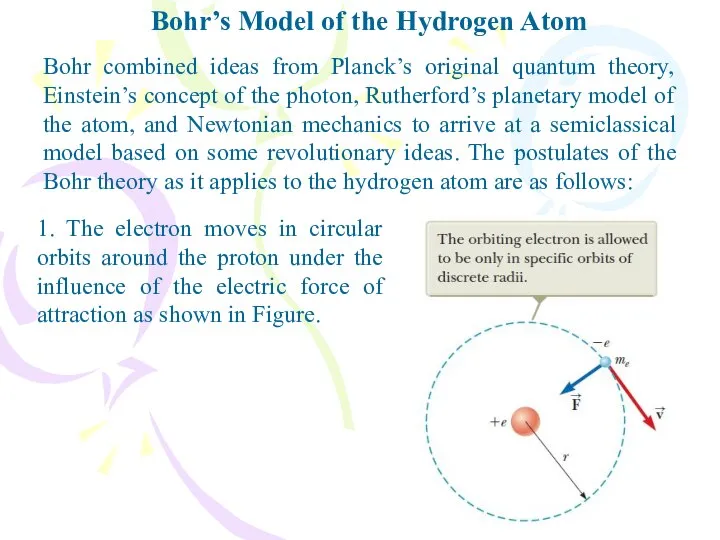
- 9. Bohr’s Model of the Hydrogen Atom Bohr combined ideas from Planck’s original quantum theory, Einstein’s concept
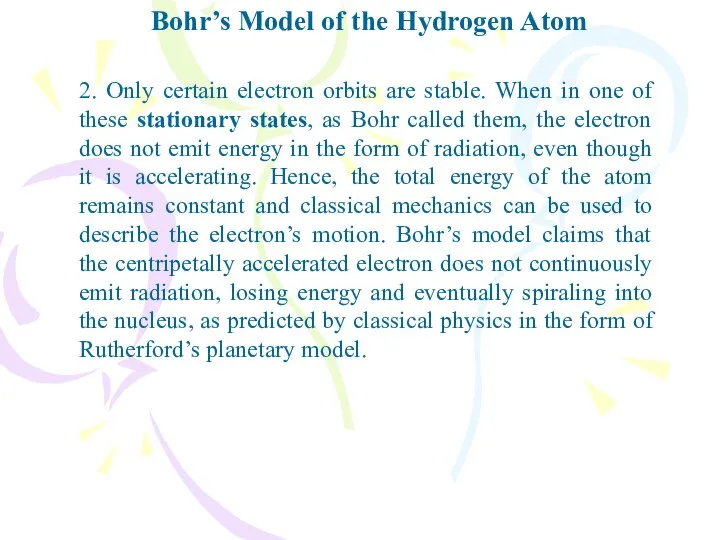
- 10. Bohr’s Model of the Hydrogen Atom 2. Only certain electron orbits are stable. When in one
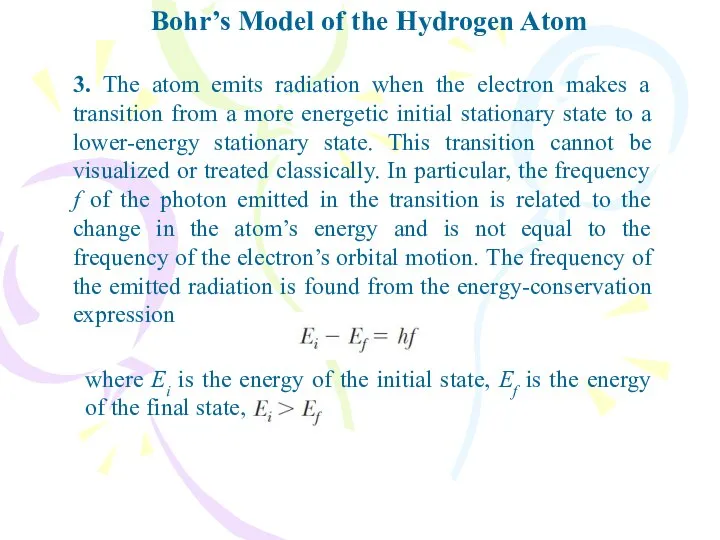
- 11. Bohr’s Model of the Hydrogen Atom 3. The atom emits radiation when the electron makes a
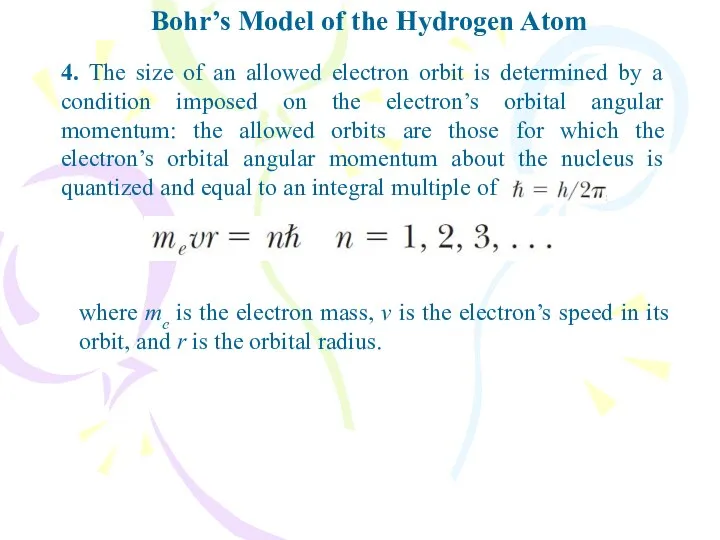
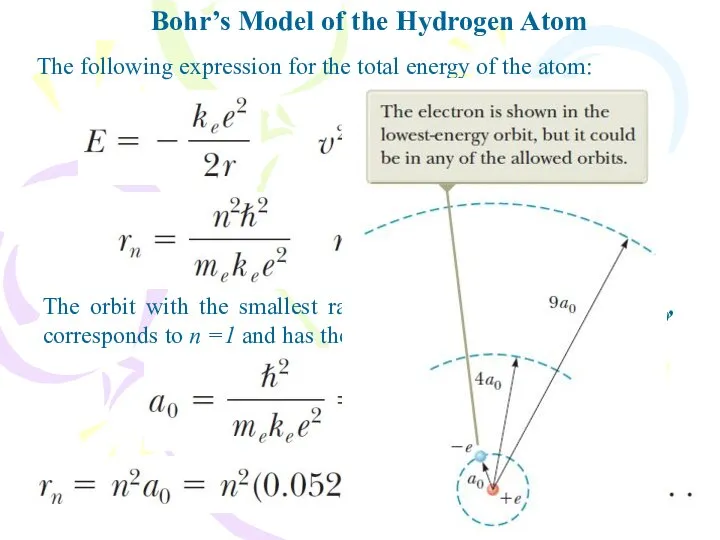
- 12. Bohr’s Model of the Hydrogen Atom 4. The size of an allowed electron orbit is determined
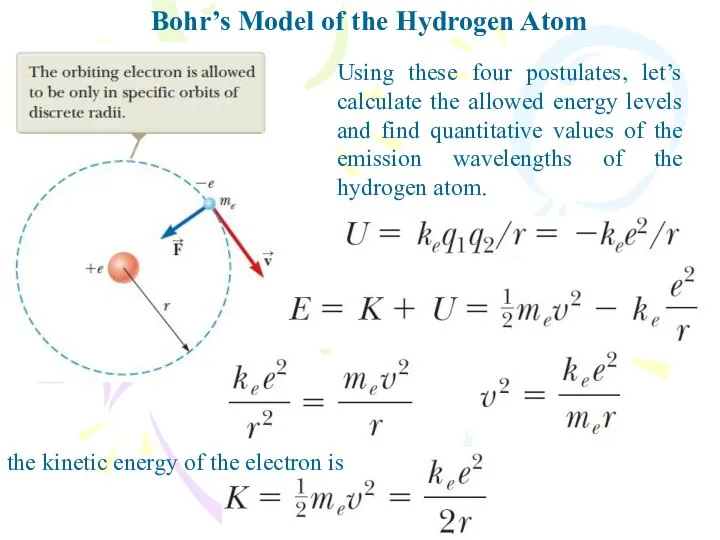
- 13. Bohr’s Model of the Hydrogen Atom Using these four postulates, let’s calculate the allowed energy levels
- 14. Bohr’s Model of the Hydrogen Atom The following expression for the total energy of the atom:
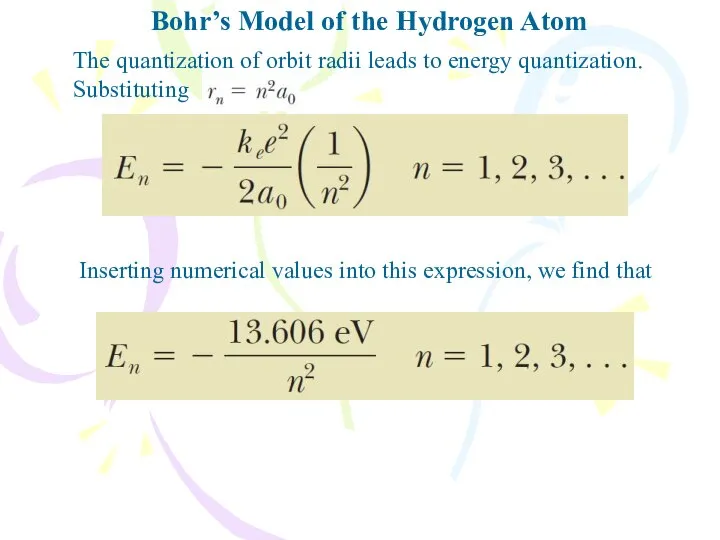
- 15. Bohr’s Model of the Hydrogen Atom The quantization of orbit radii leads to energy quantization. Substituting
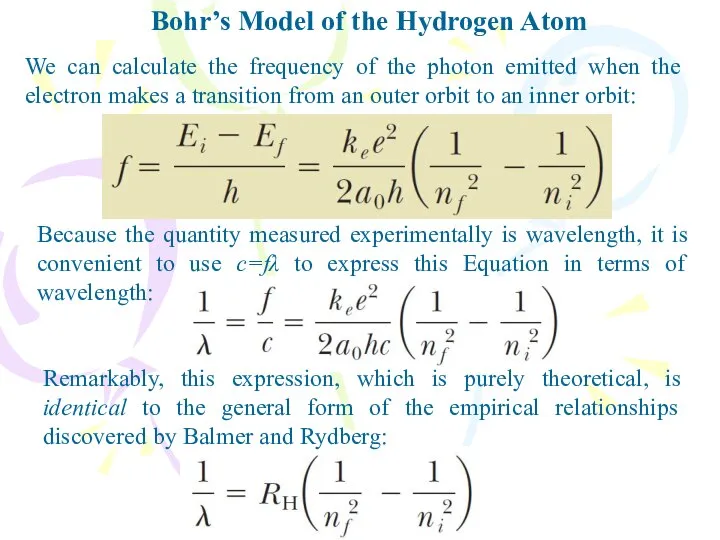
- 16. We can calculate the frequency of the photon emitted when the electron makes a transition from
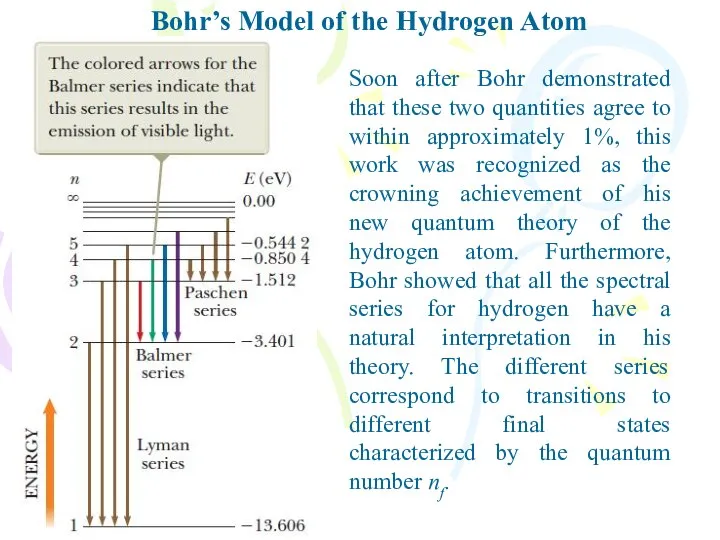
- 17. Bohr’s Model of the Hydrogen Atom Soon after Bohr demonstrated that these two quantities agree to
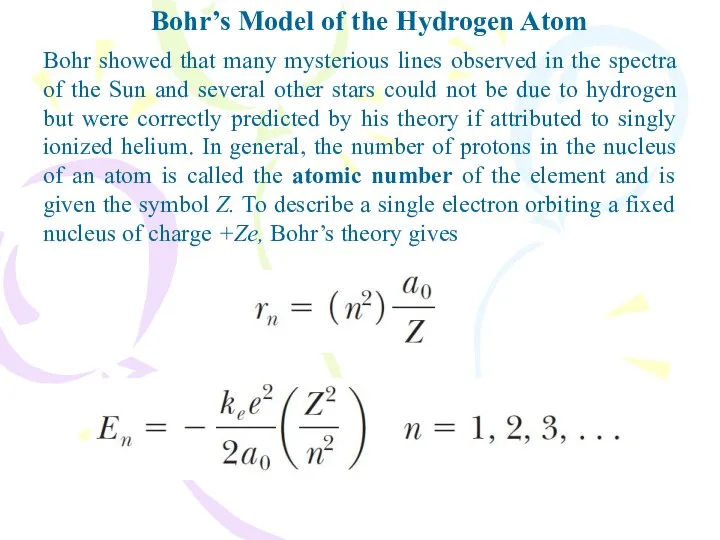
- 18. Bohr’s Model of the Hydrogen Atom Bohr showed that many mysterious lines observed in the spectra
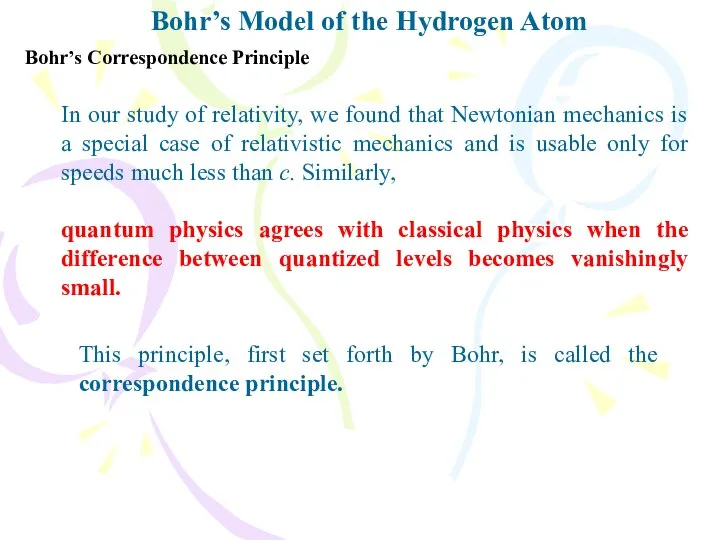
- 19. Bohr’s Model of the Hydrogen Atom Bohr’s Correspondence Principle In our study of relativity, we found
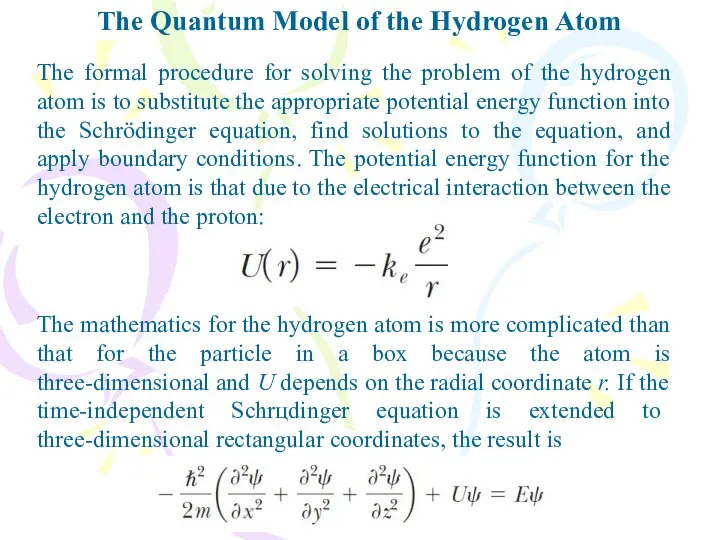
- 20. The Quantum Model of the Hydrogen Atom The formal procedure for solving the problem of the
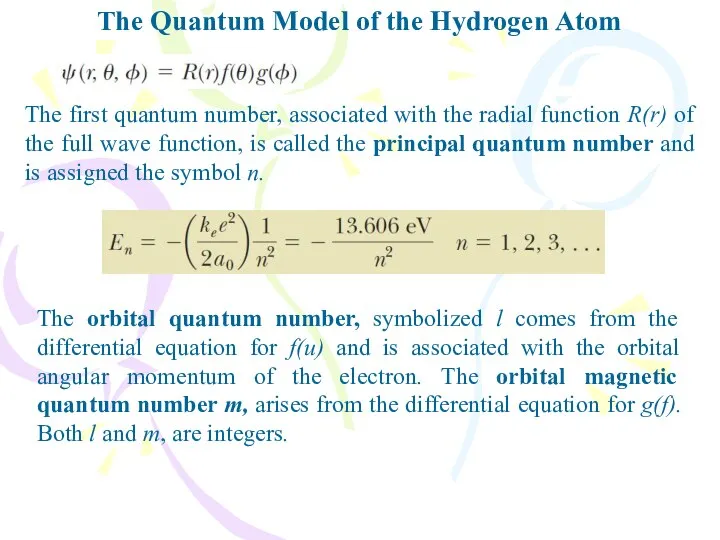
- 21. The Quantum Model of the Hydrogen Atom The first quantum number, associated with the radial function
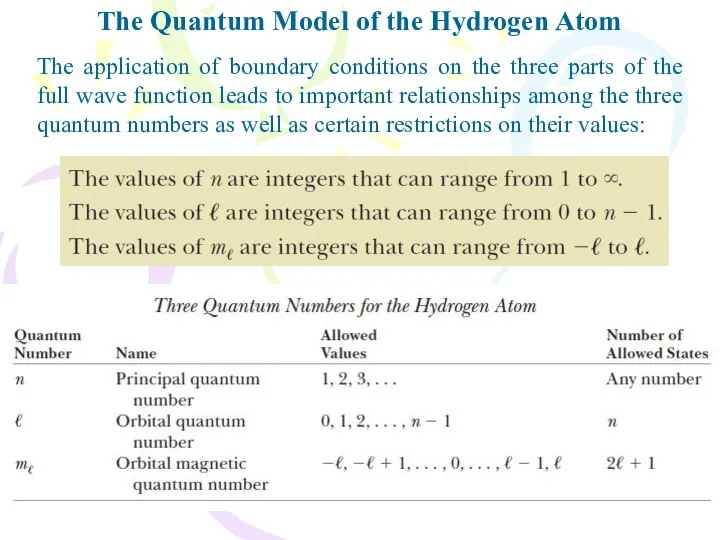
- 22. The Quantum Model of the Hydrogen Atom The application of boundary conditions on the three parts
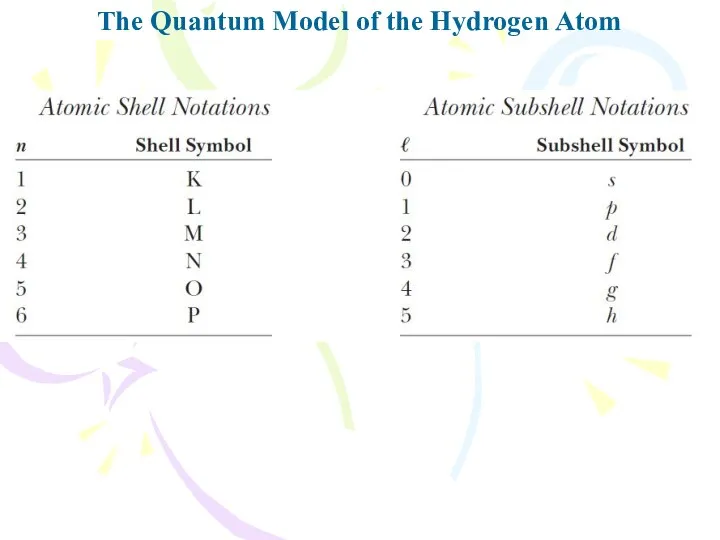
- 23. The Quantum Model of the Hydrogen Atom
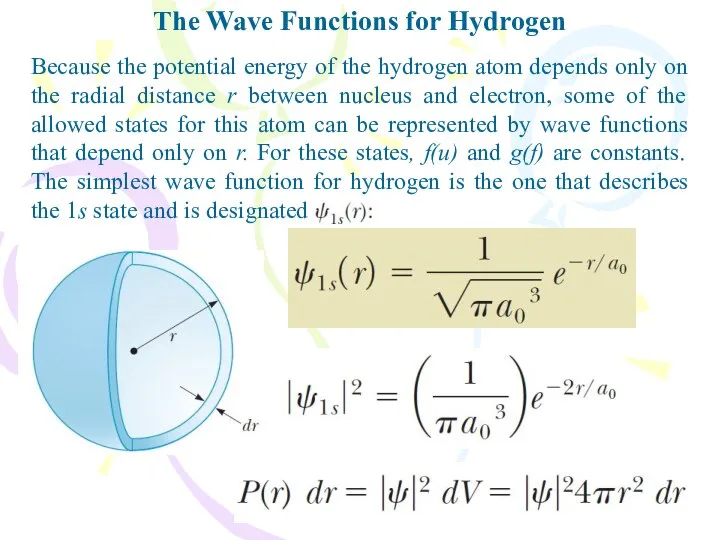
- 24. The Wave Functions for Hydrogen Because the potential energy of the hydrogen atom depends only on
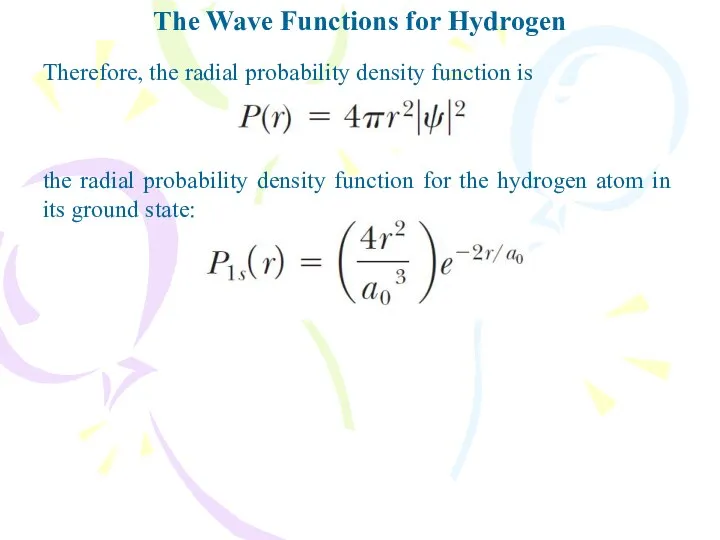
- 25. The Wave Functions for Hydrogen Therefore, the radial probability density function is the radial probability density
- 27. Скачать презентацию
 NP-складність і NP-повнота. Приклади наближених алгоритмів для NP-повних задач. Лекція 4
NP-складність і NP-повнота. Приклади наближених алгоритмів для NP-повних задач. Лекція 4 Проект модернизации системы электрохимической защиты нефтепровода
Проект модернизации системы электрохимической защиты нефтепровода Развитие студии исторического танца «Эхо времён»
Развитие студии исторического танца «Эхо времён» Понятие приватизации
Понятие приватизации Многофункциональная платформа для проектирования информационно-управляющих систем
Многофункциональная платформа для проектирования информационно-управляющих систем Презентация Нормативно-правовая база, организация управления, направления деятельности Внуковской, Домодедовской, Шереметьевско
Презентация Нормативно-правовая база, организация управления, направления деятельности Внуковской, Домодедовской, Шереметьевско Қатты дененің статикасы
Қатты дененің статикасы устные вычисления - презентация для начальной школы
устные вычисления - презентация для начальной школы Тайм - менеджмент Старт – Парк Пенза
Тайм - менеджмент Старт – Парк Пенза  Романтизм в музыке
Романтизм в музыке  Классификация морфем русского языка по значению
Классификация морфем русского языка по значению  Нормативно-правовое регулирование деятельности спортивных судей
Нормативно-правовое регулирование деятельности спортивных судей Формы залегания, структуры и текстуры
Формы залегания, структуры и текстуры сметана
сметана Титульный проект казанского сайта — это свыше 200 000 посетителей в месяц, которые просматривают свыше 1,5 млн страниц (по данным Rambler
Титульный проект казанского сайта — это свыше 200 000 посетителей в месяц, которые просматривают свыше 1,5 млн страниц (по данным Rambler  Основные типы бурильных головок для отбора керна и их конструктивные особенности. Семинар 4
Основные типы бурильных головок для отбора керна и их конструктивные особенности. Семинар 4 Контроль за режимом труда и отдыха водителей на международных перевозках
Контроль за режимом труда и отдыха водителей на международных перевозках Романовская игрушка
Романовская игрушка Қоғамдық денсаулық пен байланысты медицинаның этикалық мәселелері. Қазақстан президентінің жолдауындағы медицина тақырыбы
Қоғамдық денсаулық пен байланысты медицинаның этикалық мәселелері. Қазақстан президентінің жолдауындағы медицина тақырыбы  Спортивный стиль одежды
Спортивный стиль одежды Состав эмалей
Состав эмалей ПСИХОЛОГО-ПЕДАГОГИЧЕСКИЕ ОСНОВЫ ТРЕНЕРСКОЙ ДЕЯТЕЛЬНОСТИ 2
ПСИХОЛОГО-ПЕДАГОГИЧЕСКИЕ ОСНОВЫ ТРЕНЕРСКОЙ ДЕЯТЕЛЬНОСТИ 2 Методология исследований в менеджменте. Методология исследования управленческих ситуаций
Методология исследований в менеджменте. Методология исследования управленческих ситуаций уважаемые уважаемые коллеги!
уважаемые уважаемые коллеги! И.П.Павлов. Первый в области естественных наук
И.П.Павлов. Первый в области естественных наук Опасные и вредные производственные факторы
Опасные и вредные производственные факторы Тепловлажностный и воздушный режимы зданий
Тепловлажностный и воздушный режимы зданий Физическая культура в системе воспитания детей дошкольного возраста
Физическая культура в системе воспитания детей дошкольного возраста