Содержание
- 2. FREE ENERGY AND EQUILIBRIA ΔG ΔG > 0 reverse process is spontaneous ΔG = 0 no
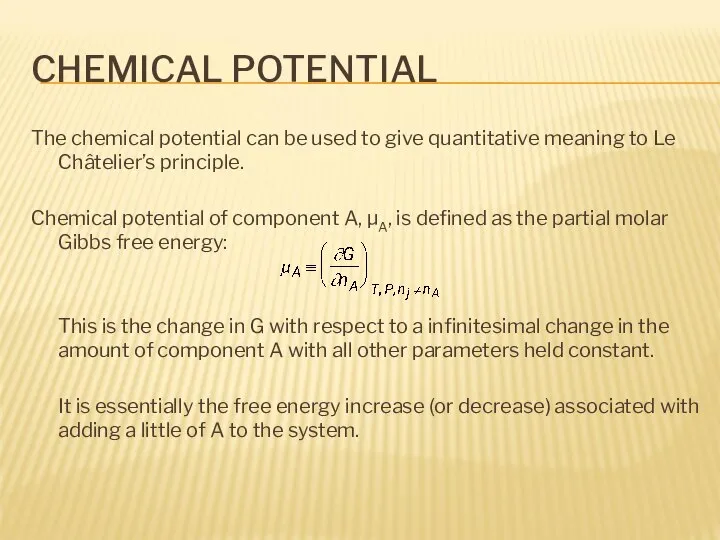
- 3. CHEMICAL POTENTIAL The chemical potential can be used to give quantitative meaning to Le Châtelier’s principle.
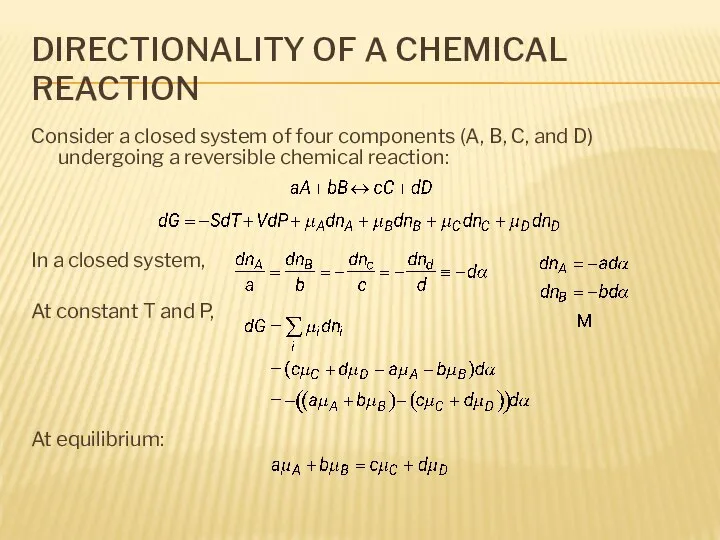
- 4. DIRECTIONALITY OF A CHEMICAL REACTION Consider a closed system of four components (A, B, C, and
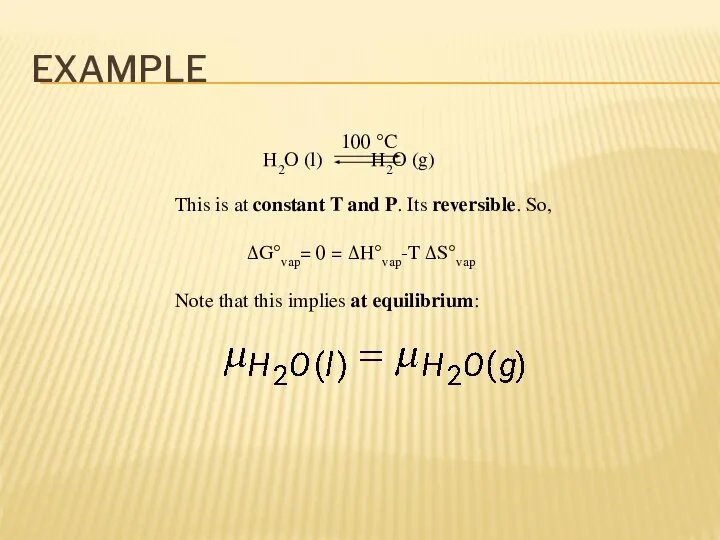
- 5. EXAMPLE H2O (l) H2O (g) 100 °C This is at constant T and P. Its reversible.
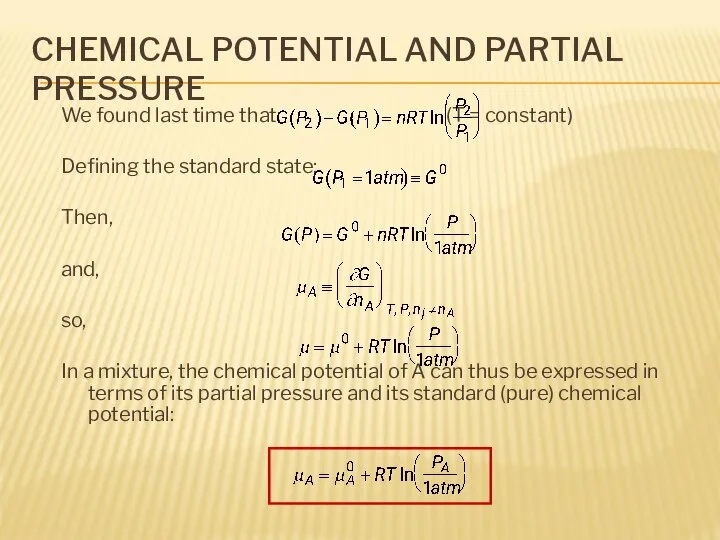
- 6. CHEMICAL POTENTIAL AND PARTIAL PRESSURE We found last time that: (T = constant) Defining the standard
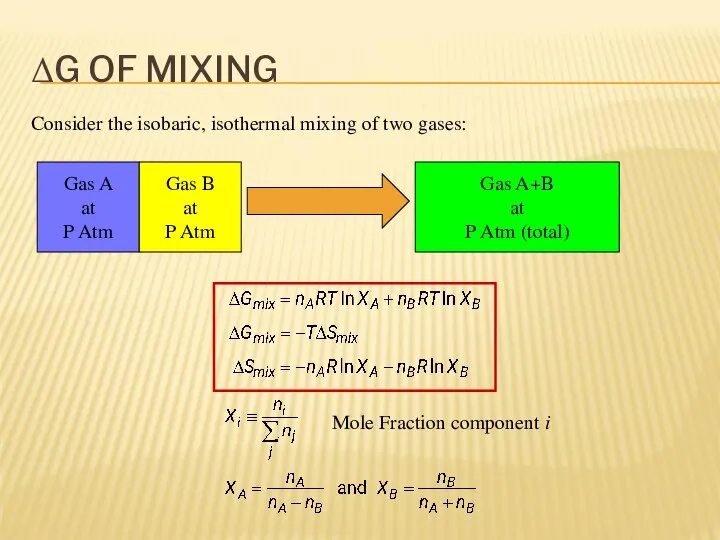
- 7. ΔG OF MIXING Consider the isobaric, isothermal mixing of two gases: Gas A at P Atm
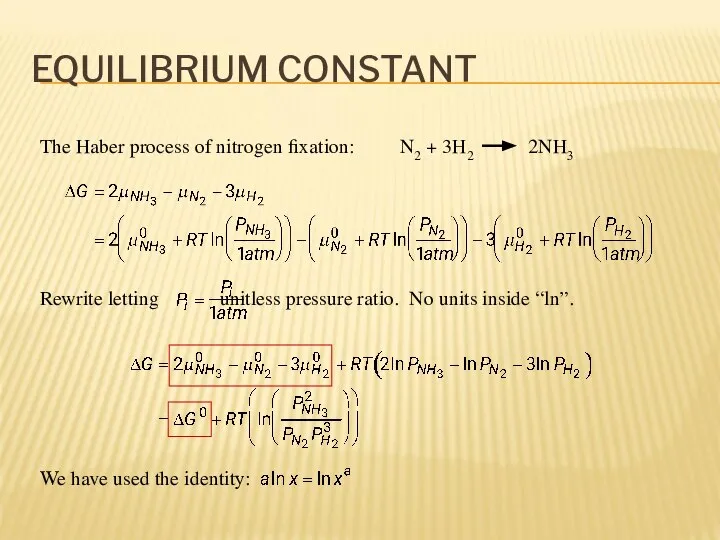
- 8. EQUILIBRIUM CONSTANT The Haber process of nitrogen fixation: N2 + 3H2 2NH3 Rewrite letting unitless pressure
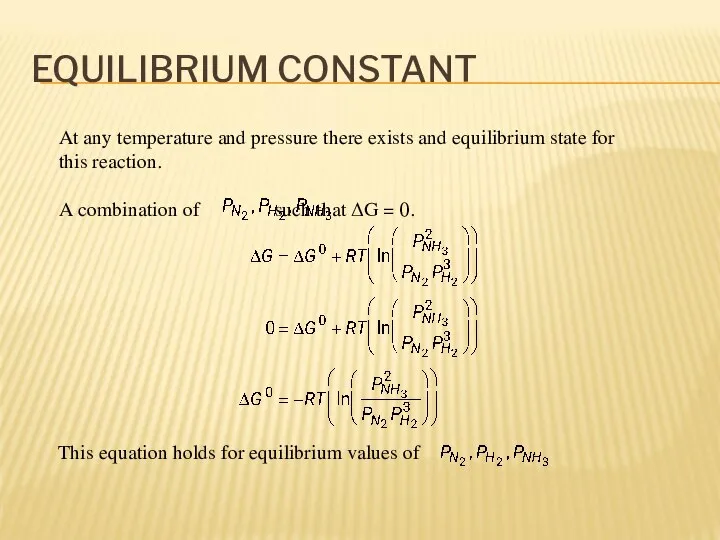
- 9. EQUILIBRIUM CONSTANT At any temperature and pressure there exists and equilibrium state for this reaction. A
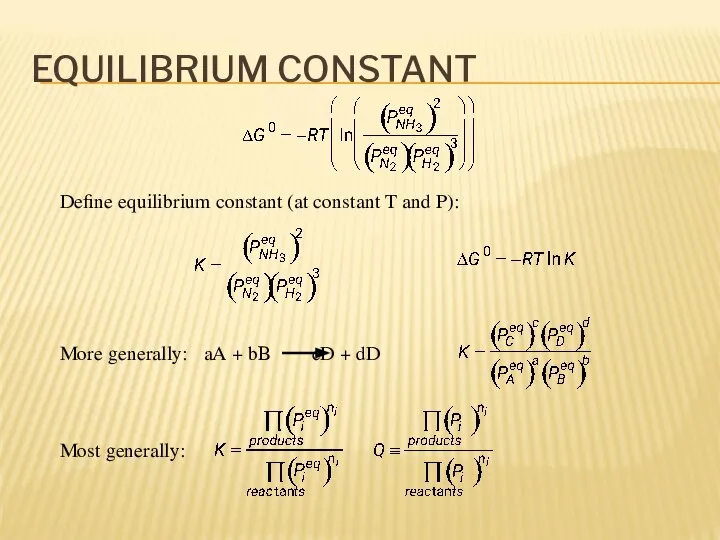
- 10. EQUILIBRIUM CONSTANT Define equilibrium constant (at constant T and P): More generally: aA + bB cD
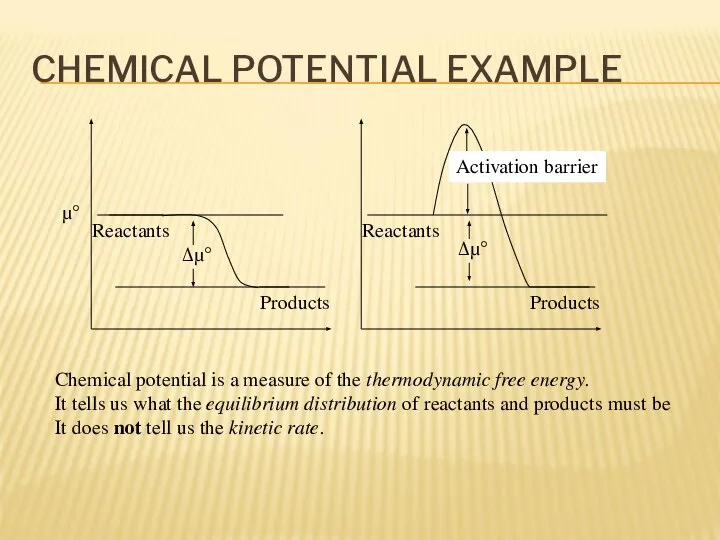
- 11. CHEMICAL POTENTIAL EXAMPLE Reactants Products Reactants Products Δμ° Δμ° μ° Chemical potential is a measure of
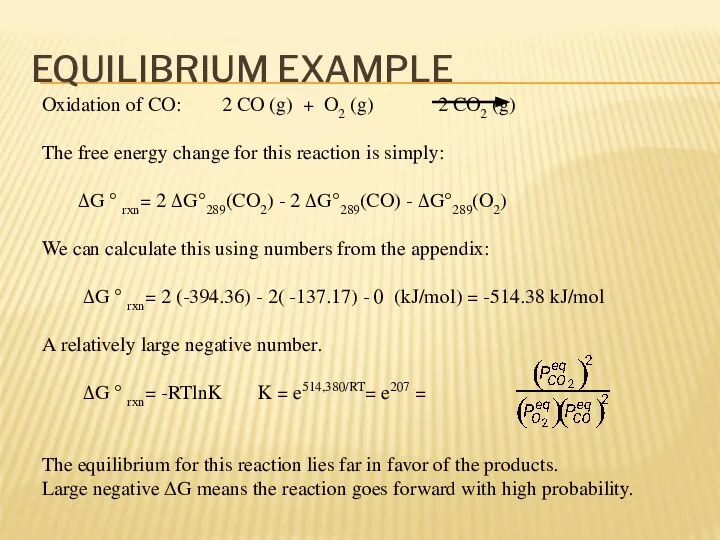
- 12. EQUILIBRIUM EXAMPLE Oxidation of CO: 2 CO (g) + O2 (g) 2 CO2 (g) The free
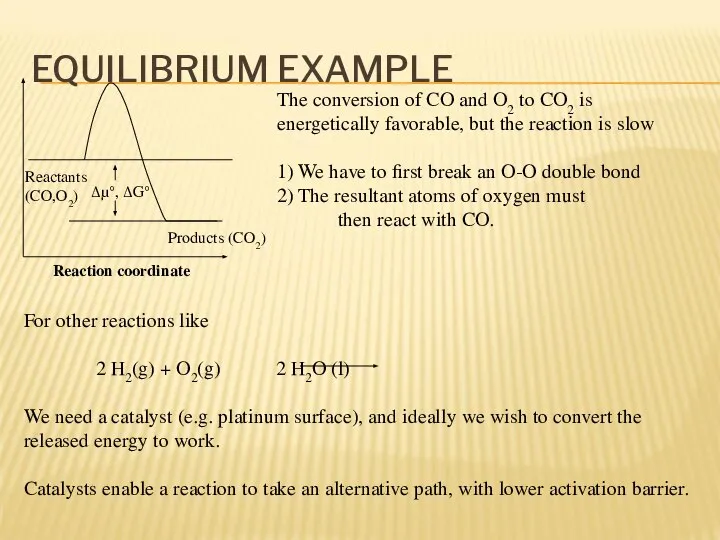
- 13. EQUILIBRIUM EXAMPLE Reactants (CO,O2) Products (CO2) Δμ°, ΔG° The conversion of CO and O2 to CO2
- 14. ΔH0 FOR SOME NONCOVALENT INTERACTIONS Na+(g) + Cl-(g) NaCl(s) Ionic -785 NaCl(s) Na+(aq) + Cl-(aq) Ionic
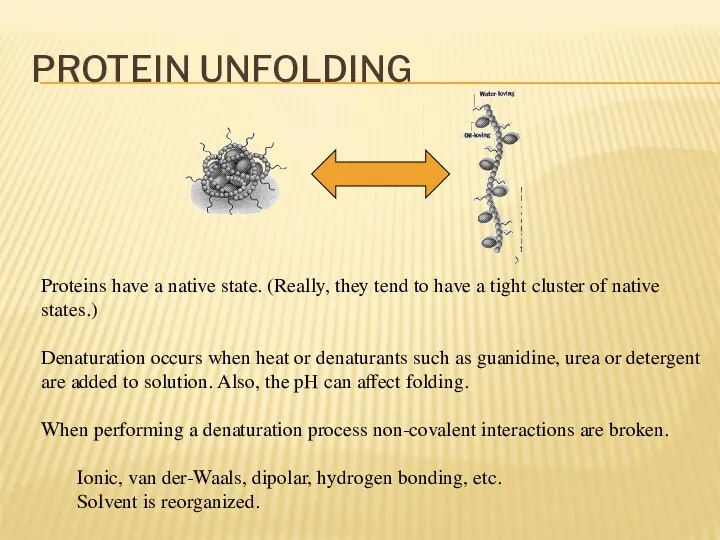
- 15. PROTEIN UNFOLDING Proteins have a native state. (Really, they tend to have a tight cluster of
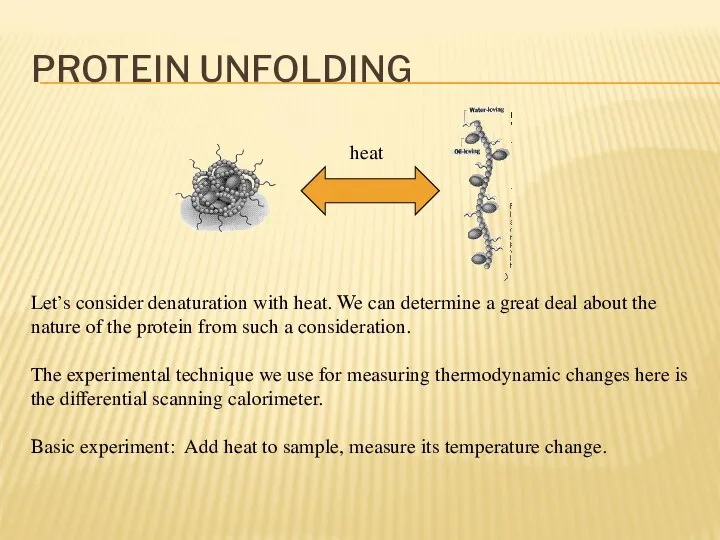
- 16. PROTEIN UNFOLDING Let’s consider denaturation with heat. We can determine a great deal about the nature
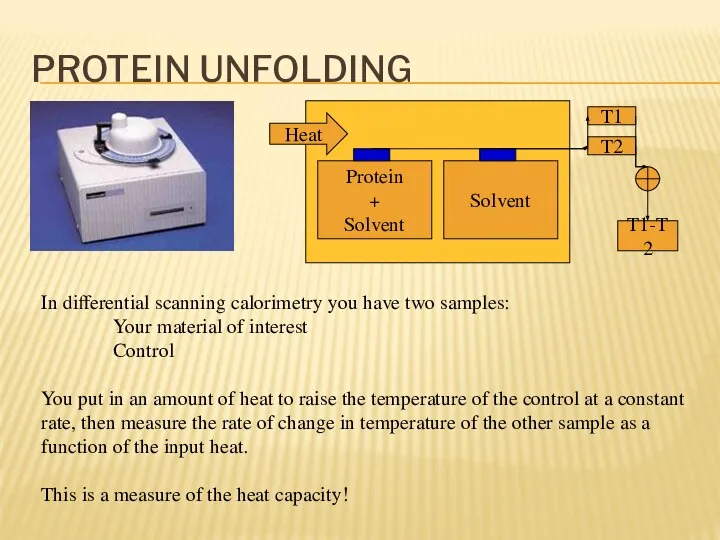
- 17. PROTEIN UNFOLDING In differential scanning calorimetry you have two samples: Your material of interest Control You
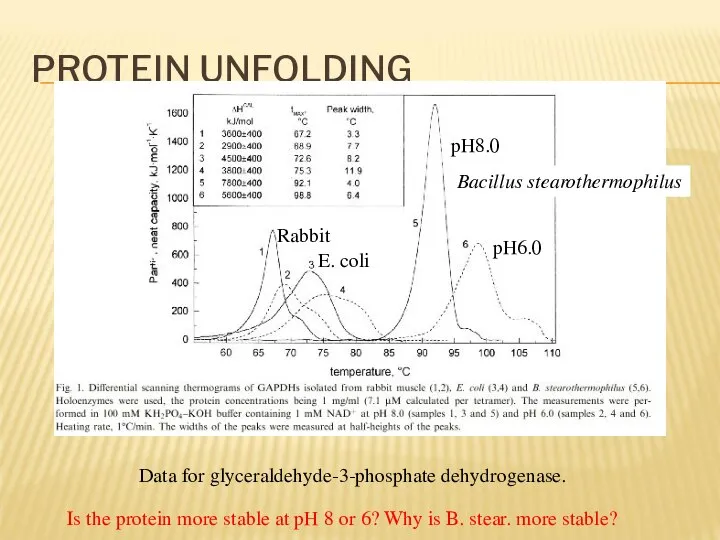
- 18. PROTEIN UNFOLDING Data for glyceraldehyde-3-phosphate dehydrogenase. pH8.0 pH6.0 Bacillus stearothermophilus E. coli Rabbit Is the protein
- 20. Скачать презентацию


 Регуляция соматических и вегетативных функций
Регуляция соматических и вегетативных функций  Підготувала учениця 10 – Б класу Краснопольська Ольга
Підготувала учениця 10 – Б класу Краснопольська Ольга Презентация Наука в современном обществе
Презентация Наука в современном обществе Виды управления в менеджменте гостеприимства
Виды управления в менеджменте гостеприимства Формы демократического участия граждан в общественной жизни Республики Беларусь
Формы демократического участия граждан в общественной жизни Республики Беларусь Презентация Организационная структура таможни
Презентация Организационная структура таможни Гомеостаз гемостаза
Гомеостаз гемостаза Структура правовой системы
Структура правовой системы Презентация на тему Кисломолочные продукты
Презентация на тему Кисломолочные продукты  Сравнение Xbox One и Sony PlayStation 4
Сравнение Xbox One и Sony PlayStation 4 Город-курорт Сочи
Город-курорт Сочи Режим и условия отбывания наказания в виде лишения свободы в исправительных учреждениях
Режим и условия отбывания наказания в виде лишения свободы в исправительных учреждениях Зимние олимпийские игры в 2018 году
Зимние олимпийские игры в 2018 году Protosoology Модификация
Protosoology Модификация Жыл басы - Наурыз
Жыл басы - Наурыз Черепно–мозговая травма Лекция
Черепно–мозговая травма Лекция Методика поверки
Методика поверки Газоны. Структура газонного покрытия. Износоустойчивость. Типы травосмесей и их значение. Классификация газонов
Газоны. Структура газонного покрытия. Износоустойчивость. Типы травосмесей и их значение. Классификация газонов La cuestión de España, y la discusión sobre el carácter nacional en la literatura periodística de la primera mitad del siglo
La cuestión de España, y la discusión sobre el carácter nacional en la literatura periodística de la primera mitad del siglo Логическое программирование (Prolog)
Логическое программирование (Prolog) Ливарні шедеври
Ливарні шедеври Цицерон. Политические взгляды. Петрова М.А. М-111б.
Цицерон. Политические взгляды. Петрова М.А. М-111б. Управление решениями
Управление решениями  Презентация по тенденциям и особенностям пространственного развития городов
Презентация по тенденциям и особенностям пространственного развития городов Статистическое управление процессами
Статистическое управление процессами Правила игры в регби
Правила игры в регби Языки программирования высокого уровня
Языки программирования высокого уровня Экологическая экспертиза
Экологическая экспертиза