Содержание
- 2. Course of lectures «Contemporary Physics: Part1» Lecture №8 The Kinetic Theory of Gases. Heat Engines, Entropy,
- 3. Molecular Model of an Ideal Gas The macroscopic description of model of ideal gas make the
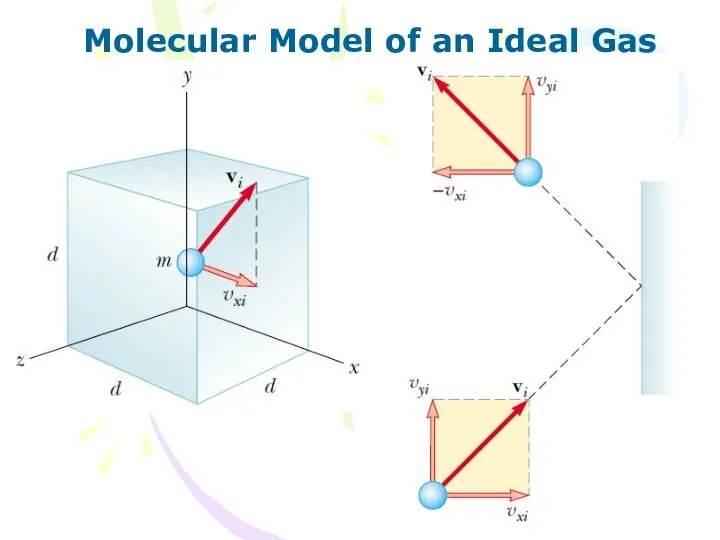
- 4. Molecular Model of an Ideal Gas
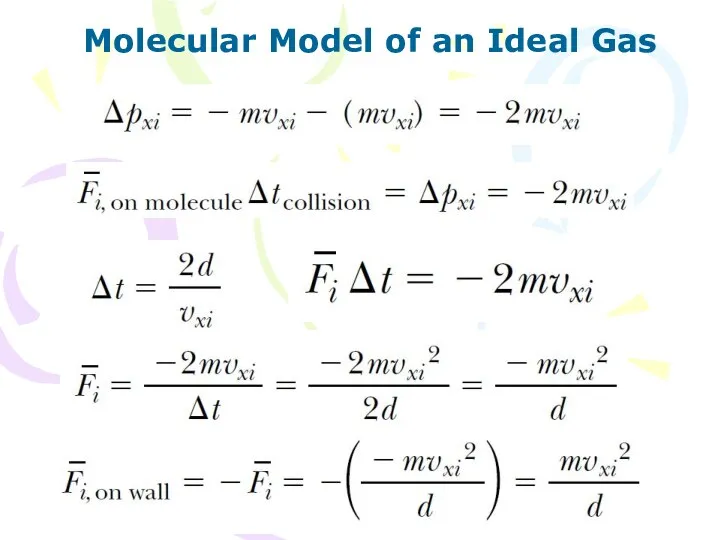
- 5. Molecular Model of an Ideal Gas
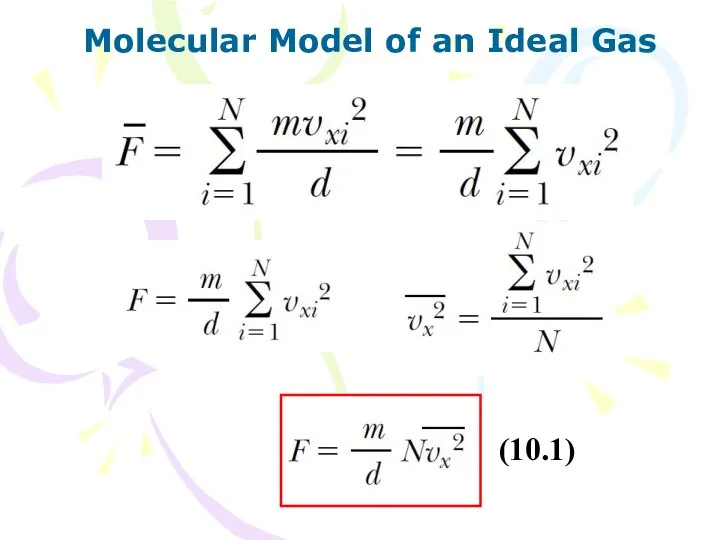
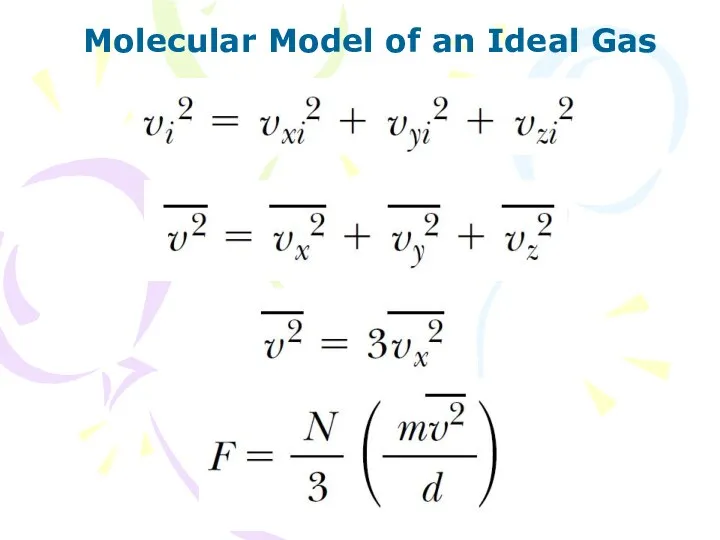
- 6. Molecular Model of an Ideal Gas (10.1)
- 7. Molecular Model of an Ideal Gas
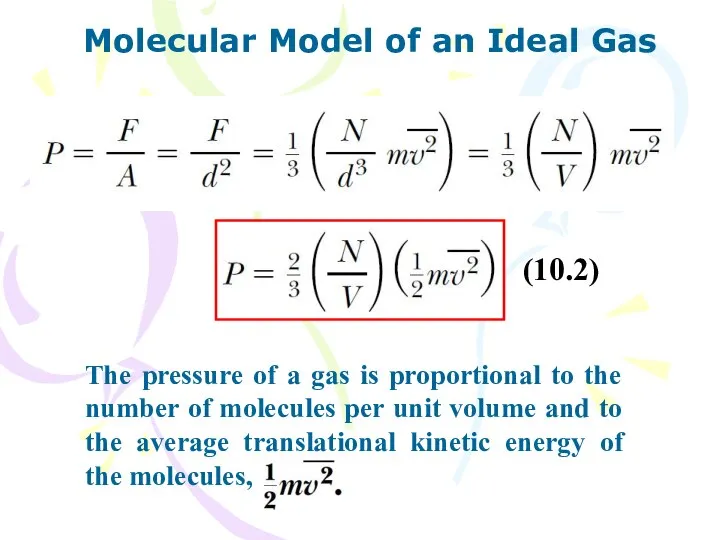
- 8. Molecular Model of an Ideal Gas (10.2) The pressure of a gas is proportional to the
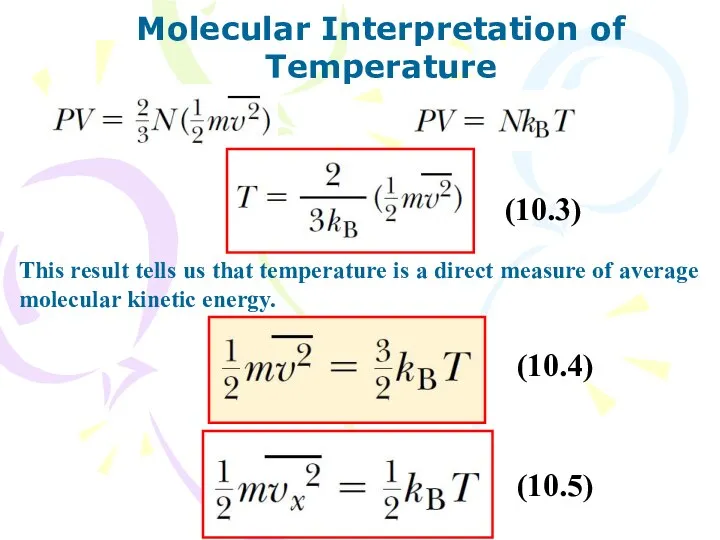
- 9. Molecular Interpretation of Temperature This result tells us that temperature is a direct measure of average
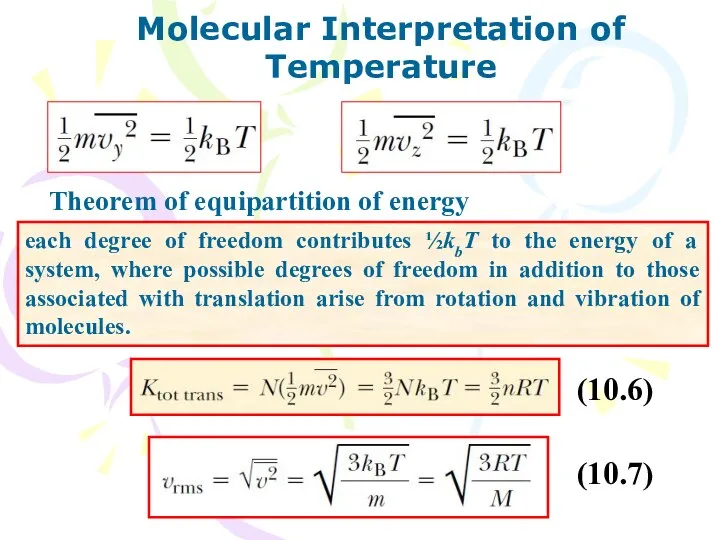
- 10. Molecular Interpretation of Temperature Theorem of equipartition of energy each degree of freedom contributes ½kbT to
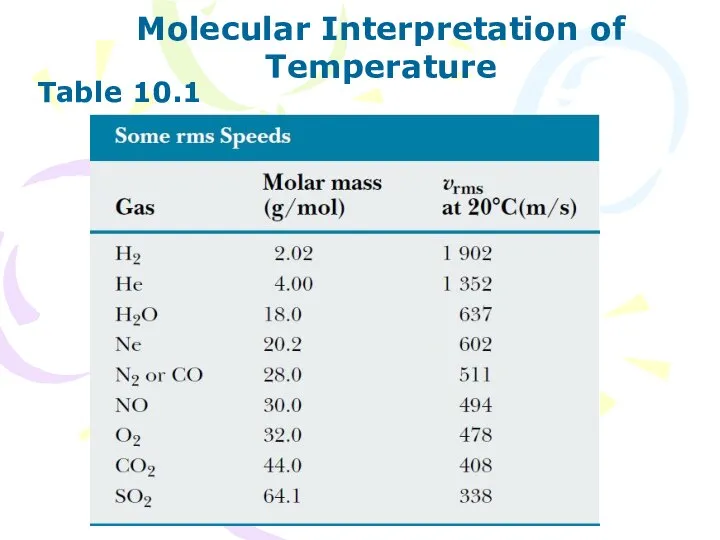
- 11. Molecular Interpretation of Temperature Table 10.1
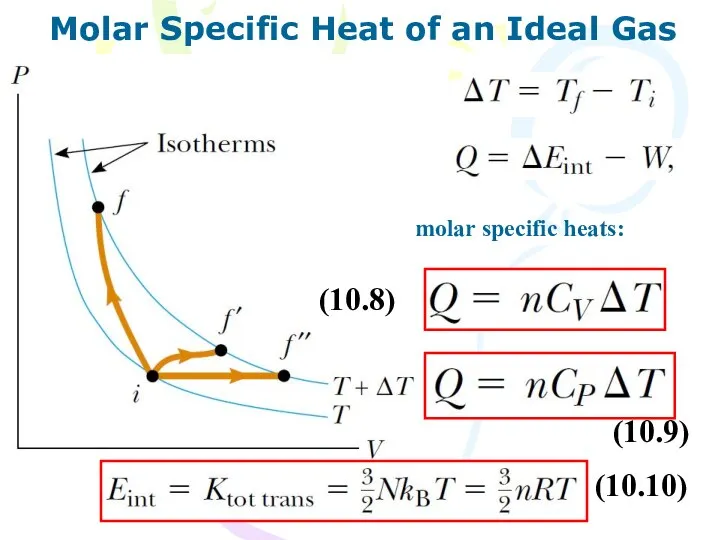
- 12. Molar Specific Heat of an Ideal Gas molar specific heats: (10.8) (10.9) (10.10)
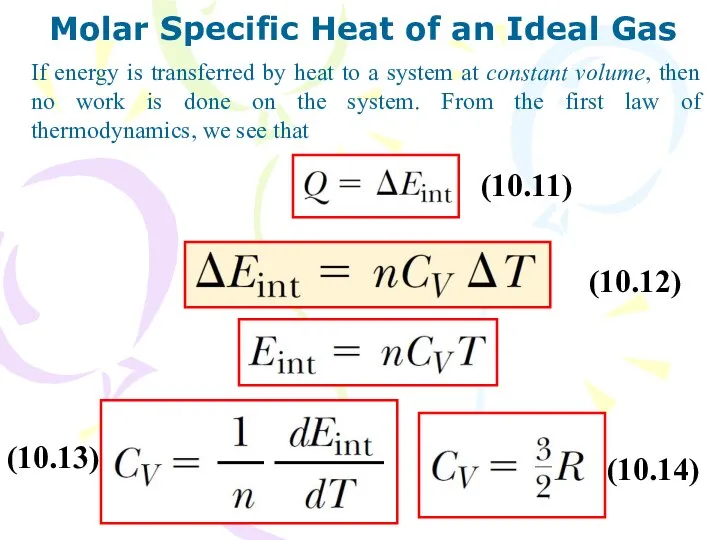
- 13. Molar Specific Heat of an Ideal Gas If energy is transferred by heat to a system
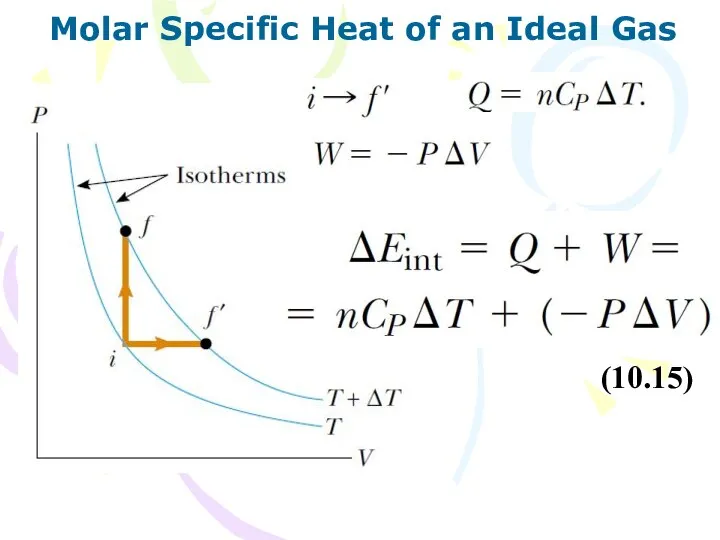
- 14. Molar Specific Heat of an Ideal Gas (10.15)
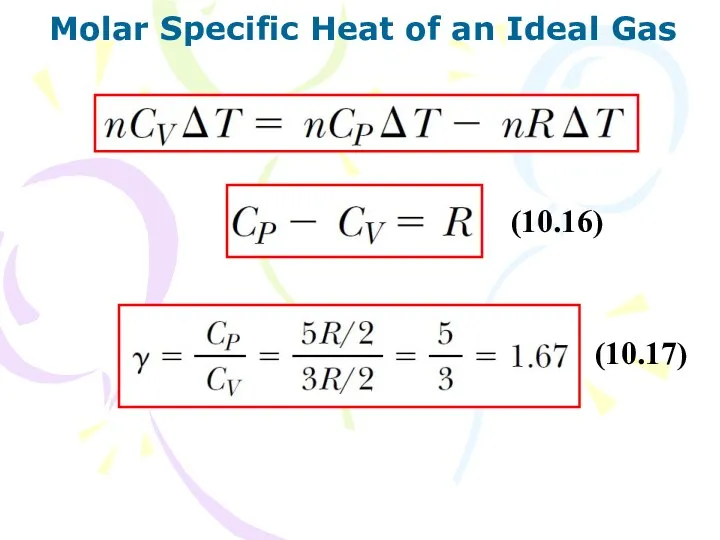
- 15. Molar Specific Heat of an Ideal Gas (10.16) (10.17)
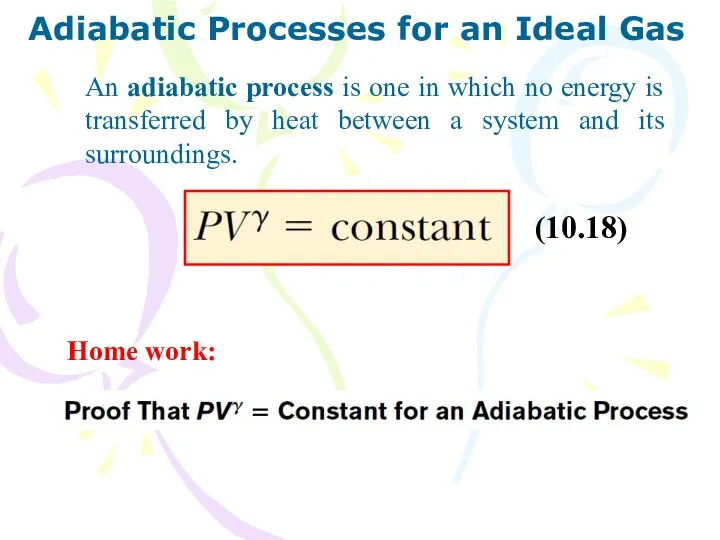
- 16. Adiabatic Processes for an Ideal Gas An adiabatic process is one in which no energy is
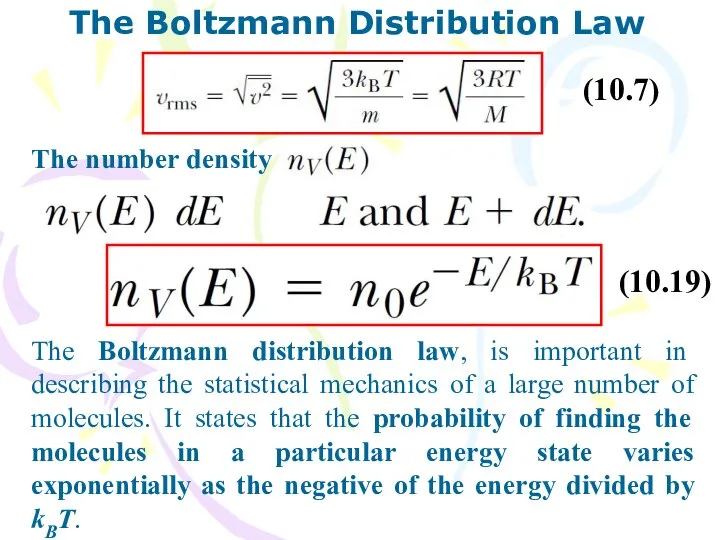
- 17. The Boltzmann Distribution Law (10.7) The number density (10.19) The Boltzmann distribution law, is important in
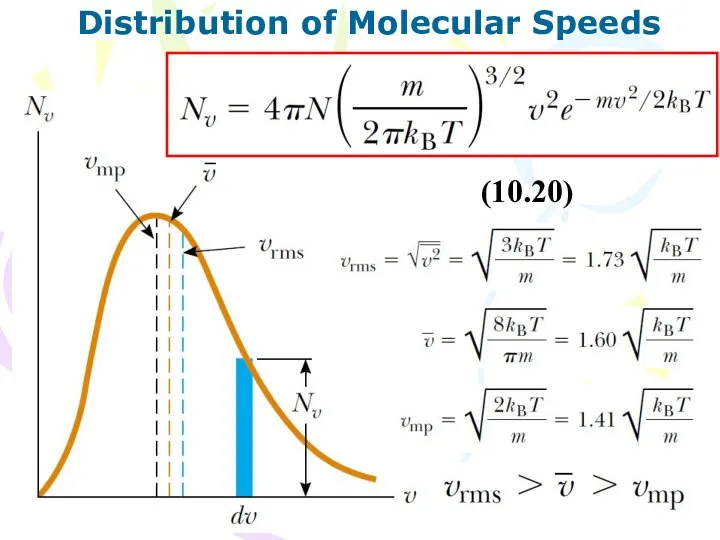
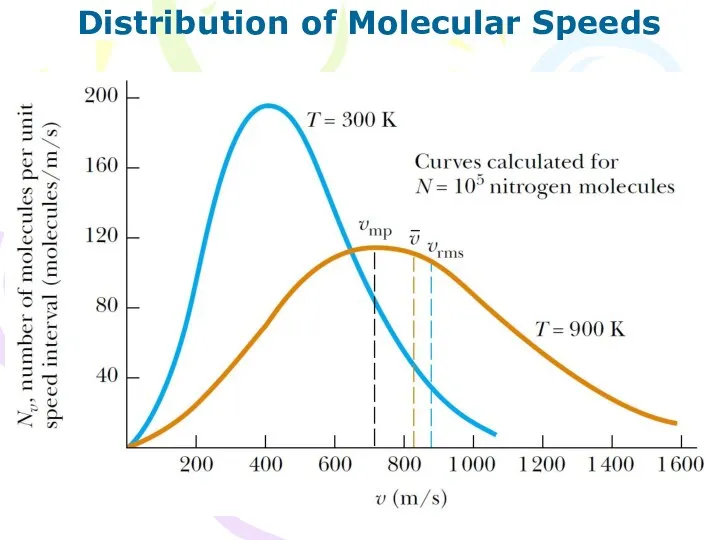
- 18. Distribution of Molecular Speeds (10.20)
- 19. Distribution of Molecular Speeds
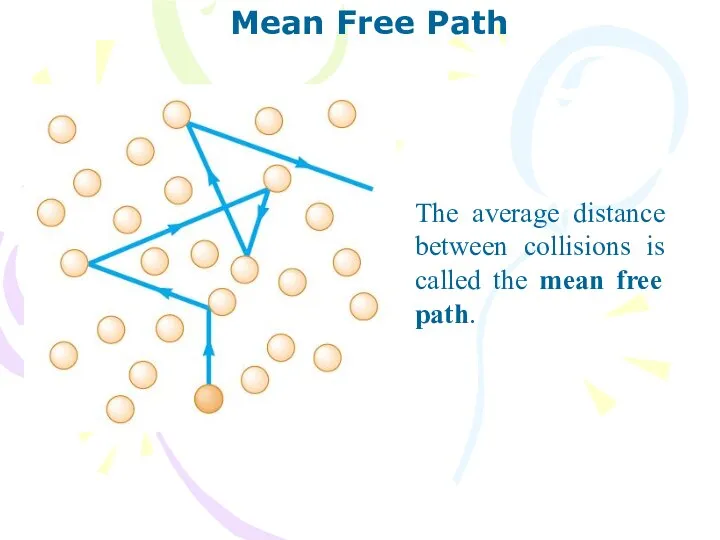
- 20. Mean Free Path The average distance between collisions is called the mean free path.
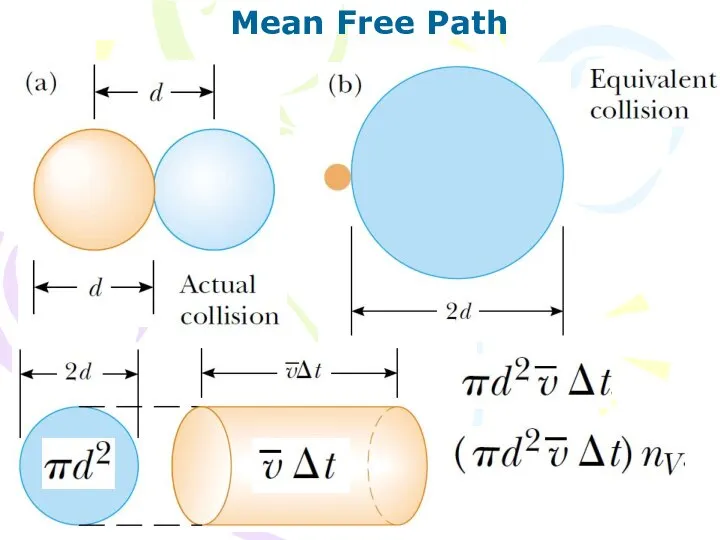
- 21. Mean Free Path
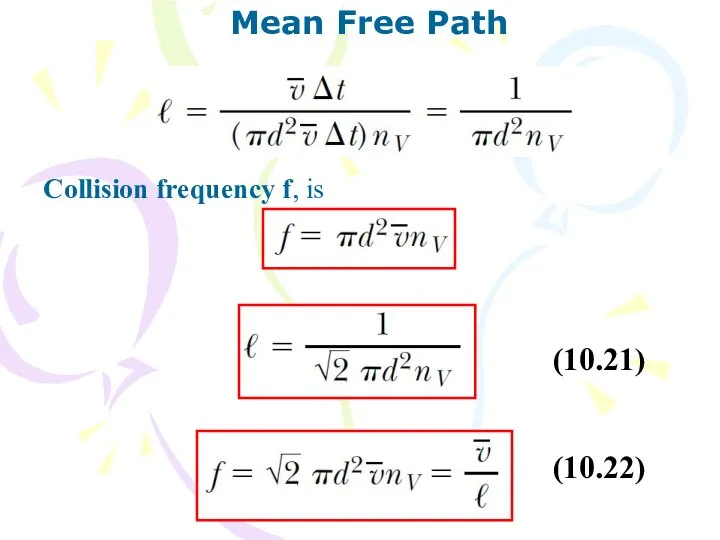
- 22. Mean Free Path (10.21) (10.22) Collision frequency f, is
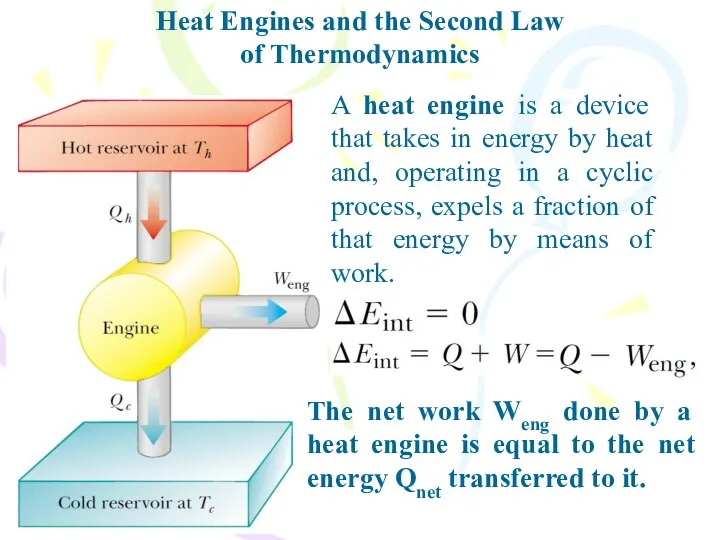
- 23. Heat Engines and the Second Law of Thermodynamics A heat engine is a device that takes
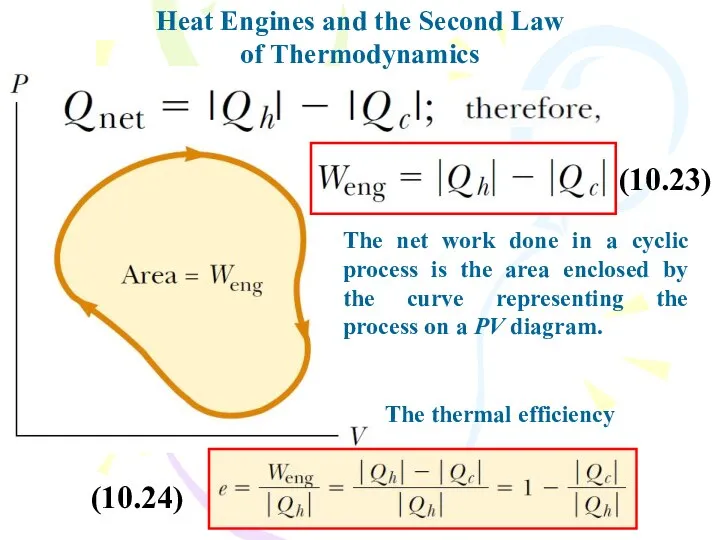
- 24. Heat Engines and the Second Law of Thermodynamics The net work done in a cyclic process
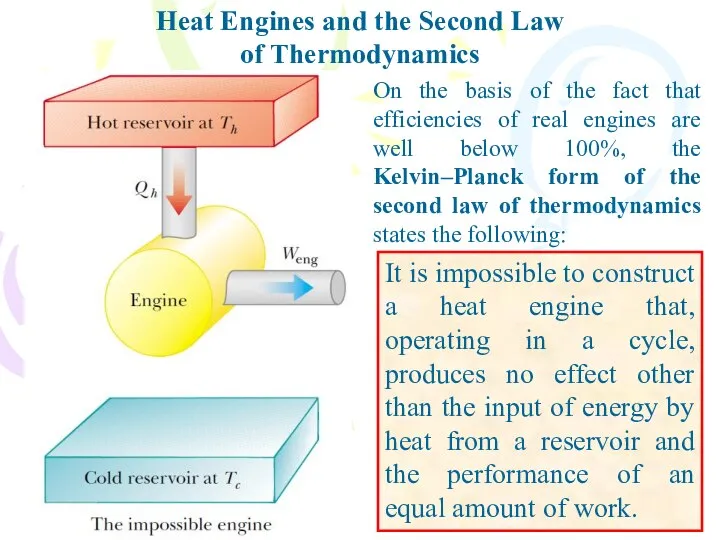
- 25. On the basis of the fact that efficiencies of real engines are well below 100%, the
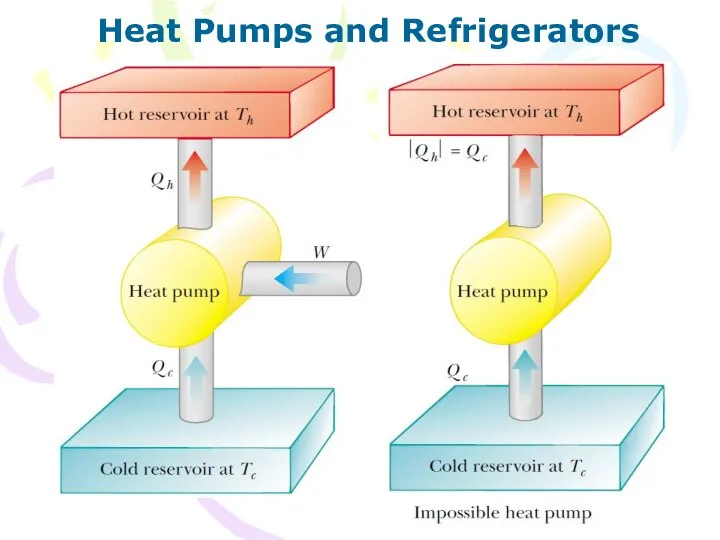
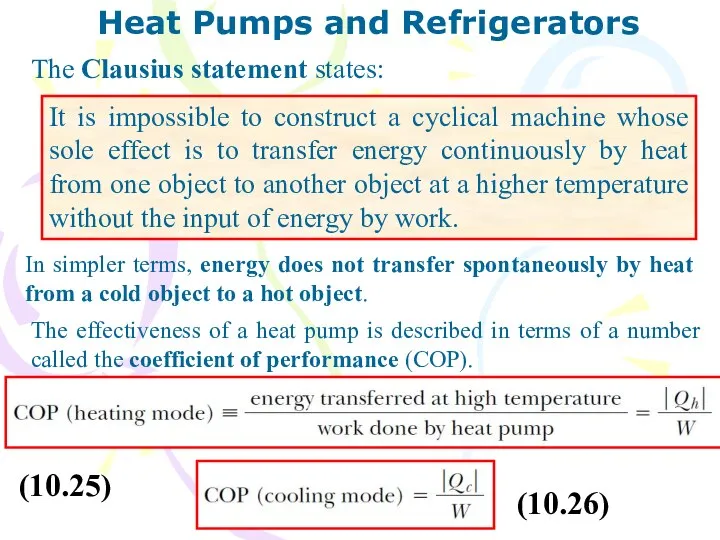
- 26. Heat Pumps and Refrigerators
- 27. Heat Pumps and Refrigerators The Clausius statement states: It is impossible to construct a cyclical machine
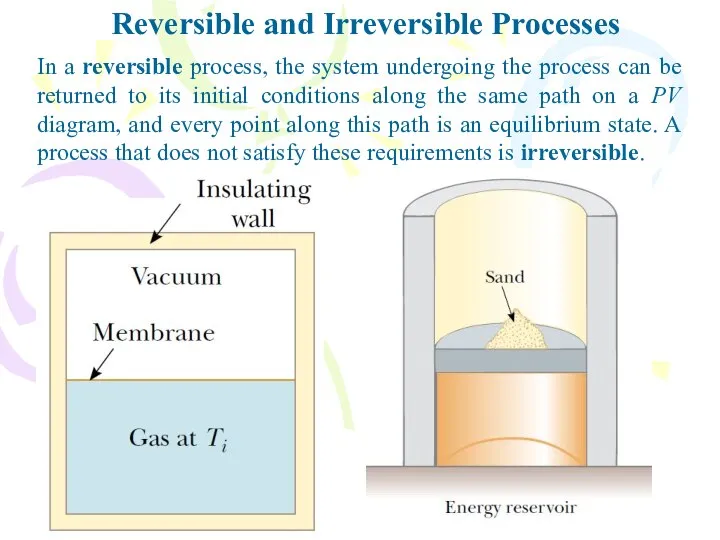
- 28. Reversible and Irreversible Processes In a reversible process, the system undergoing the process can be returned
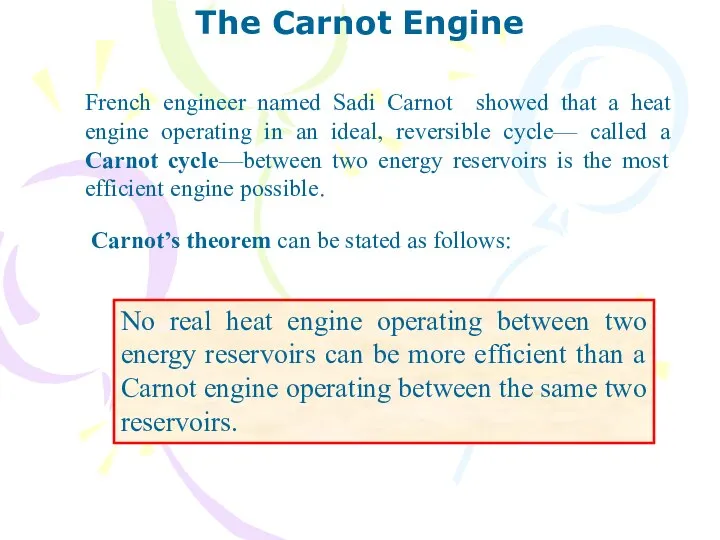
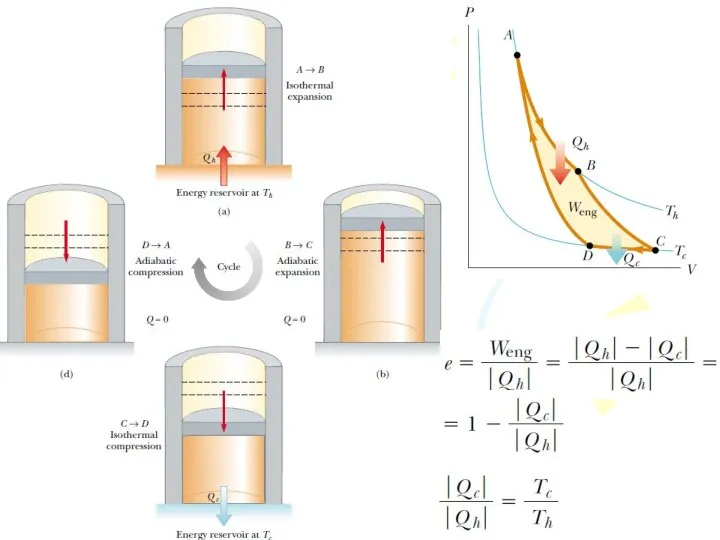
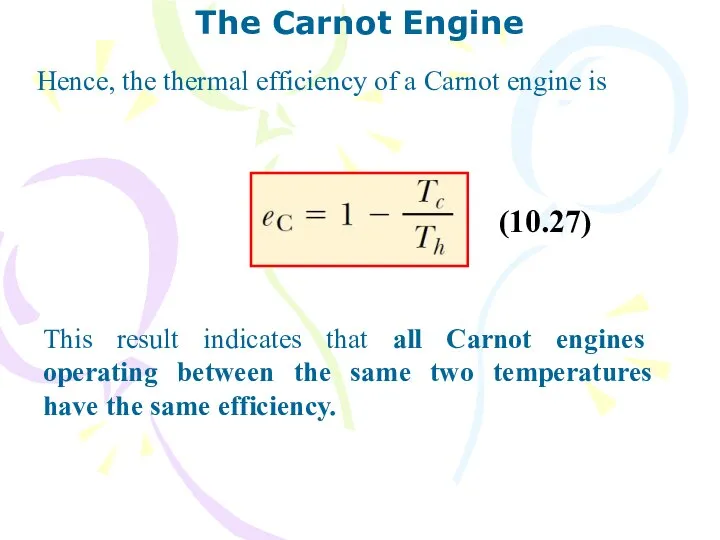
- 29. The Carnot Engine French engineer named Sadi Carnot showed that a heat engine operating in an
- 31. The Carnot Engine This result indicates that all Carnot engines operating between the same two temperatures
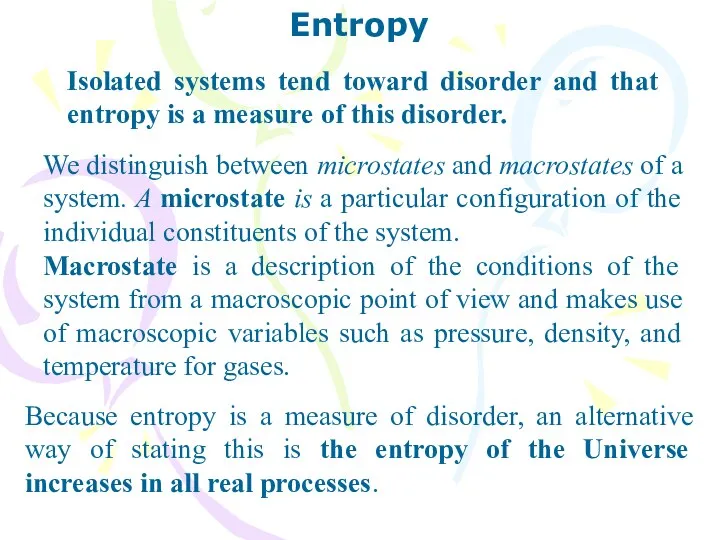
- 32. Entropy Isolated systems tend toward disorder and that entropy is a measure of this disorder. We
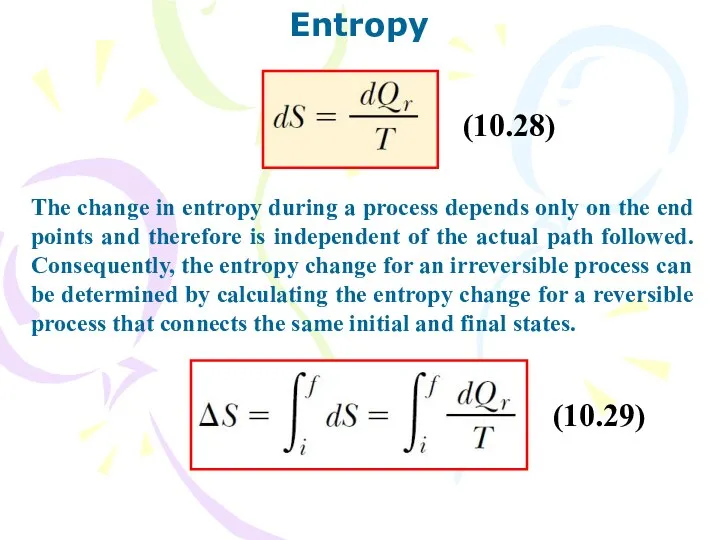
- 33. Entropy The change in entropy during a process depends only on the end points and therefore
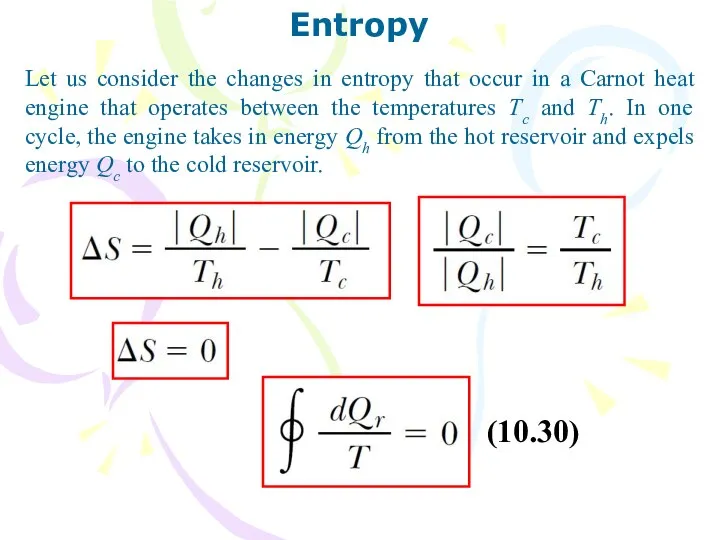
- 34. Entropy Let us consider the changes in entropy that occur in a Carnot heat engine that
- 35. Quick Quiz 5.3 If you are asked to make a very sensitive glass thermometer, which of
- 37. Скачать презентацию


 Теневая экономика в современных условиях. Выполнила: Учащаяся экономического факультета Гринева Наталия группы Э091
Теневая экономика в современных условиях. Выполнила: Учащаяся экономического факультета Гринева Наталия группы Э091 Мехатронные системы в различных сферах производственной деятельности
Мехатронные системы в различных сферах производственной деятельности Методология исследований в менеджменте и маркетинге
Методология исследований в менеджменте и маркетинге Презентация "Теория спроса и предложения. Равновесие на рынке" - скачать презентации по Экономике
Презентация "Теория спроса и предложения. Равновесие на рынке" - скачать презентации по Экономике Молодежное открытое правительство
Молодежное открытое правительство 79842
79842 Мотивация учения и ее формирование у учащихся начальной школы. ДЕНИСОВА В.А.
Мотивация учения и ее формирование у учащихся начальной школы. ДЕНИСОВА В.А.  Башкиры. Народы Поволжья
Башкиры. Народы Поволжья Программирование на языке ассемблер
Программирование на языке ассемблер Масленица
Масленица Диагностика систем управления
Диагностика систем управления Великий Леонардо да Винчи
Великий Леонардо да Винчи Оперативный ток
Оперативный ток Проектирование здания учебно-тренажерного комплекса Саратовского подразделения Приволжского УЦПК
Проектирование здания учебно-тренажерного комплекса Саратовского подразделения Приволжского УЦПК Оценка сложности алгоритмов
Оценка сложности алгоритмов Презентация Товары к которым применяются запреты или ограничения на ввоз или вывоз
Презентация Товары к которым применяются запреты или ограничения на ввоз или вывоз Нуклеофильное замещение у винильного атома углерода
Нуклеофильное замещение у винильного атома углерода
 СТУПЕНЬКИ К ШКОЛЕ ПСИХОЛОГО-ПЕДАГОГИЧЕСКАЯ ГОТОВНОСТЬ РЕБЕНКА К ШКОЛЕ
СТУПЕНЬКИ К ШКОЛЕ ПСИХОЛОГО-ПЕДАГОГИЧЕСКАЯ ГОТОВНОСТЬ РЕБЕНКА К ШКОЛЕ Новый год в Китае
Новый год в Китае Диаграмма классов UML. The Unified Modeling Language
Диаграмма классов UML. The Unified Modeling Language Организация и проведение криминологического исследования
Организация и проведение криминологического исследования Обычаи, обряды и традиции русского народа
Обычаи, обряды и традиции русского народа Есептеуіш жүйенің даму тарихы
Есептеуіш жүйенің даму тарихы Қылмыстық кодекс
Қылмыстық кодекс Вина во Франции
Вина во Франции  Мезенская роспись - презентация_
Мезенская роспись - презентация_ Презентация Основы таможенного дела
Презентация Основы таможенного дела Никола́й Ива́нович Лобаче́вский (1793-1856) - русский математик, создатель неевклидовой геометрии
Никола́й Ива́нович Лобаче́вский (1793-1856) - русский математик, создатель неевклидовой геометрии