Содержание
- 2. Lecture 6 Heat flow and the first law of thermodynamics. Kind of thermodynamic process. Adiabatic processes.
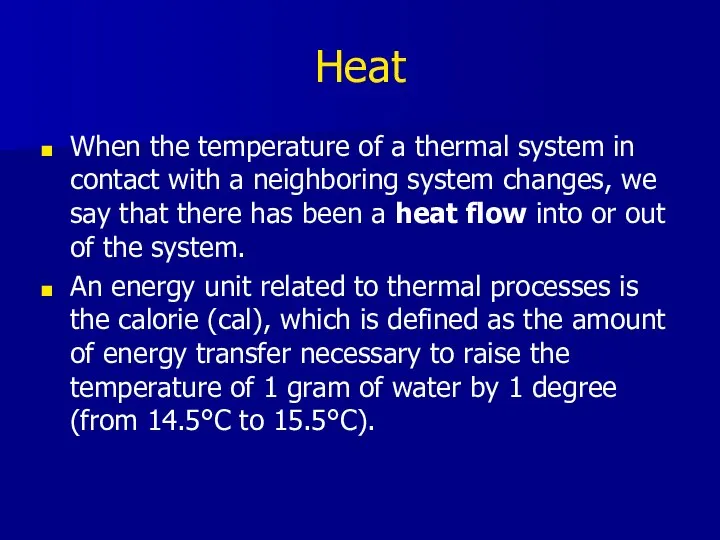
- 3. Heat When the temperature of a thermal system in contact with a neighboring system changes, we
- 4. Mechanical equivalent of heat Mechanical energy is not conserserved in the presence of nonconservative forces. It
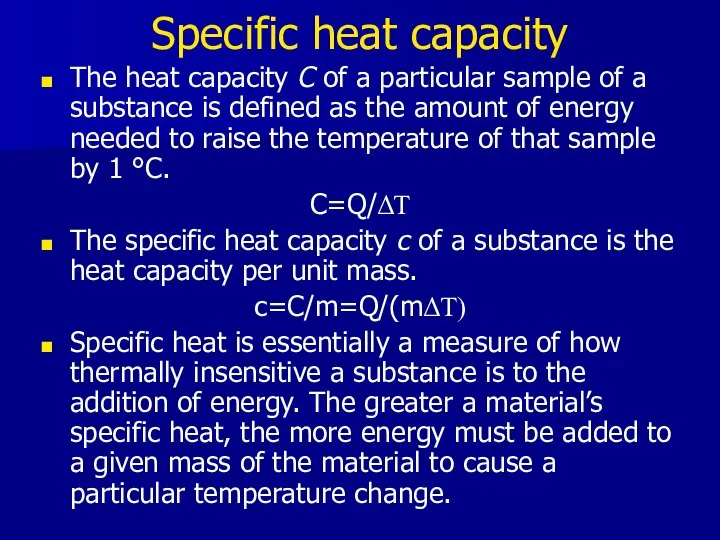
- 5. Specific heat capacity The heat capacity C of a particular sample of a substance is defined
- 6. Energy transfer and specific heat capacity From this definition, we can relate the energy Q transferred
- 8. Dependence of specific heat capacity on temperature Specific heat varies with temperature. For example, the specific
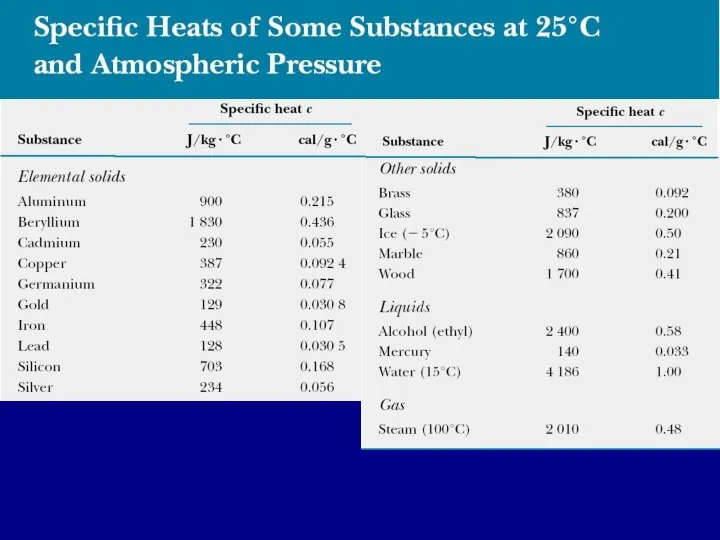
- 9. Dependence of specific heat capacity on volume and pressure Measured values of specific heats are found
- 10. Phase transition It can be that transfer of energy does not result in a change in
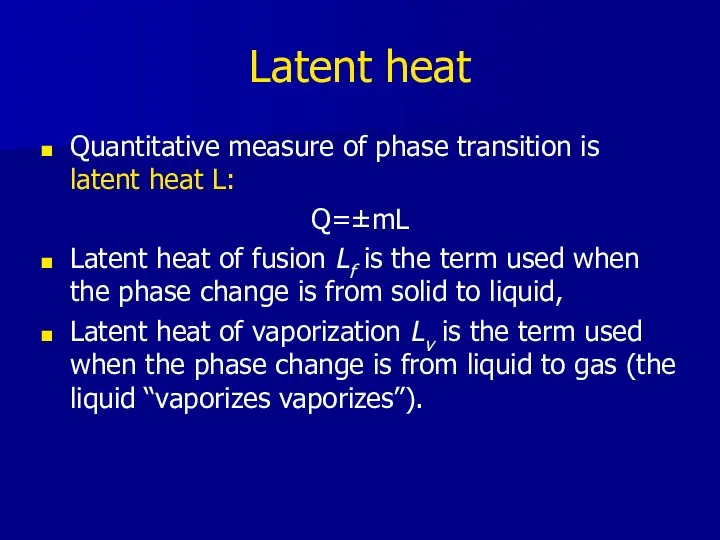
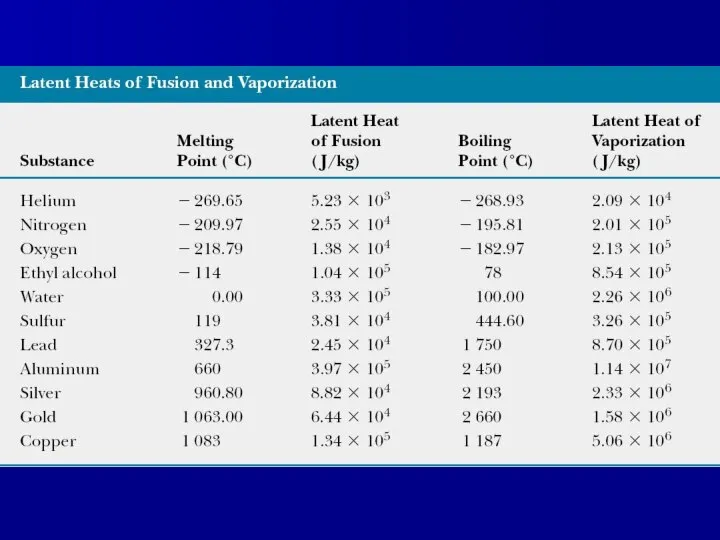
- 11. Latent heat Quantitative measure of phase transition is latent heat L: Q=±mL Latent heat of fusion
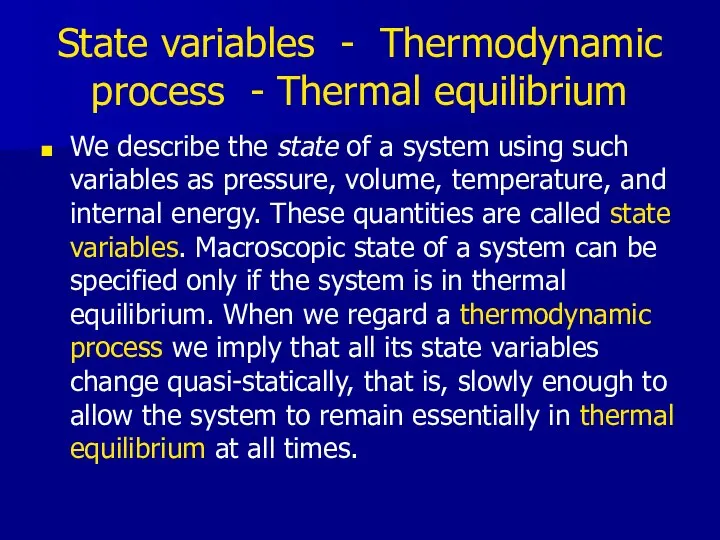
- 13. State variables - Thermodynamic process - Thermal equilibrium We describe the state of a system using
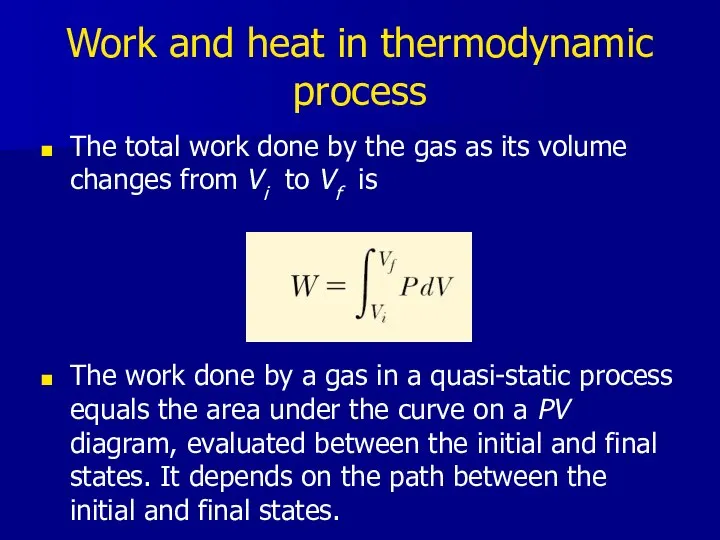
- 14. Work and heat in thermodynamic process The total work done by the gas as its volume
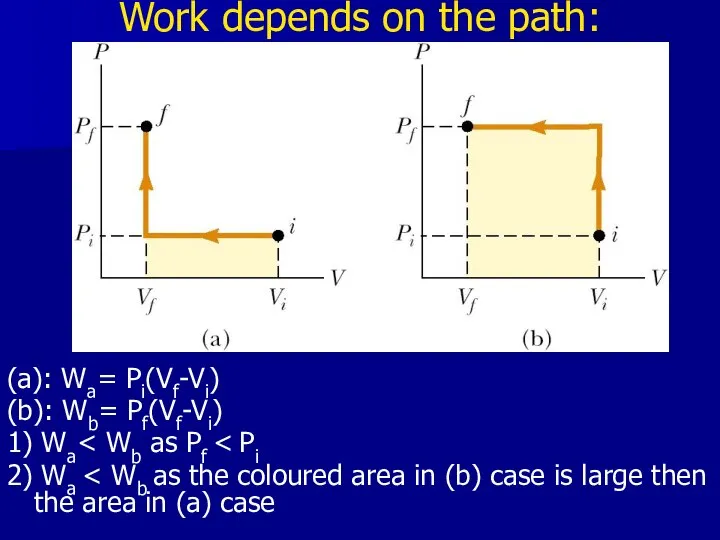
- 15. Work depends on the path: (a): Wa= Pi(Vf-Vi) (b): Wb= Pf(Vf-Vi) 1) Wa 2) Wa
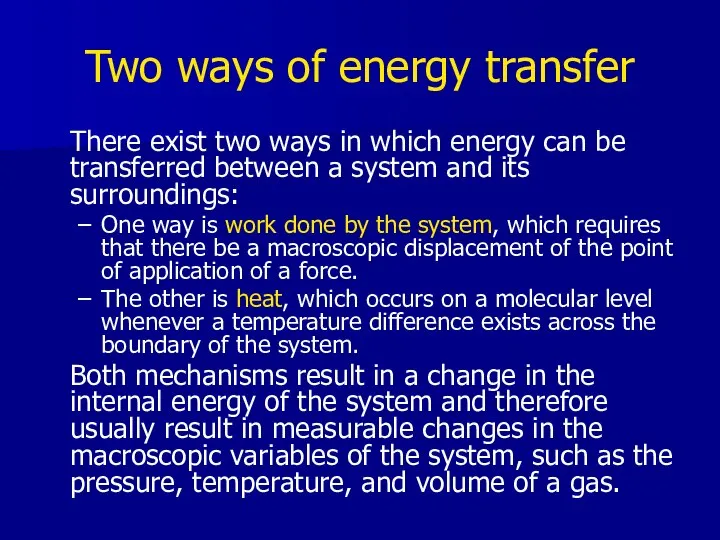
- 16. Two ways of energy transfer There exist two ways in which energy can be transferred between
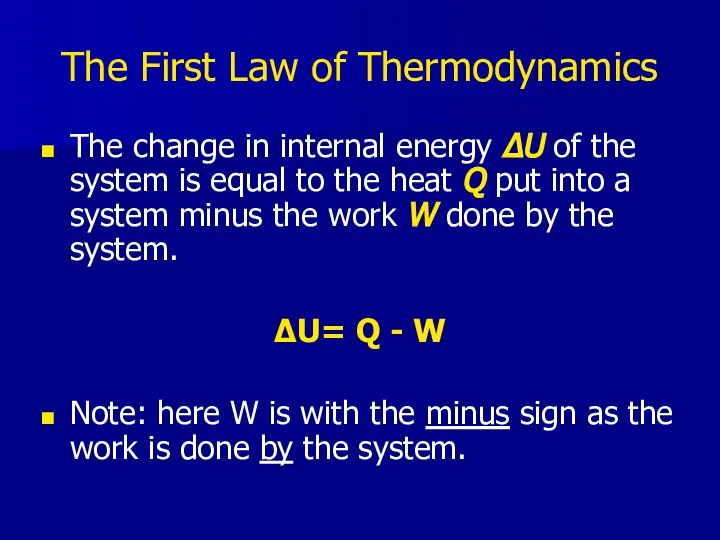
- 17. The First Law of Thermodynamics The change in internal energy ΔU of the system is equal
- 18. The first law of thermodynamics is a special case of the law of conservation of energy
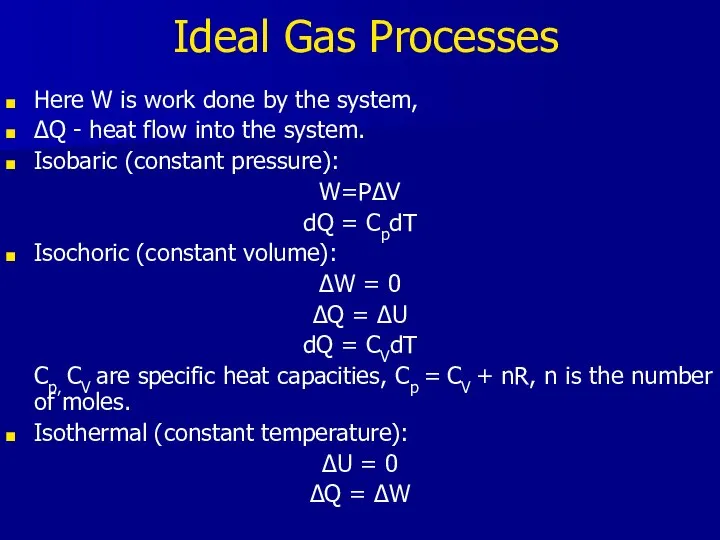
- 19. Ideal Gas Processes Here W is work done by the system, ΔQ - heat flow into
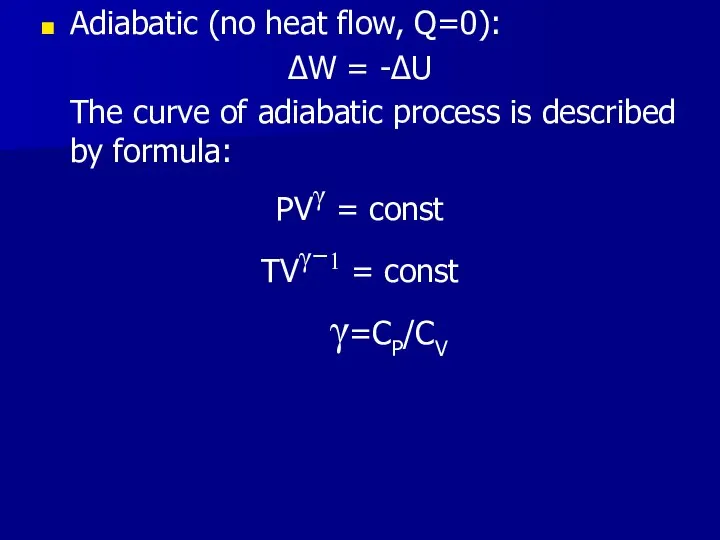
- 20. Adiabatic (no heat flow, Q=0): ΔW = -ΔU The curve of adiabatic process is described by
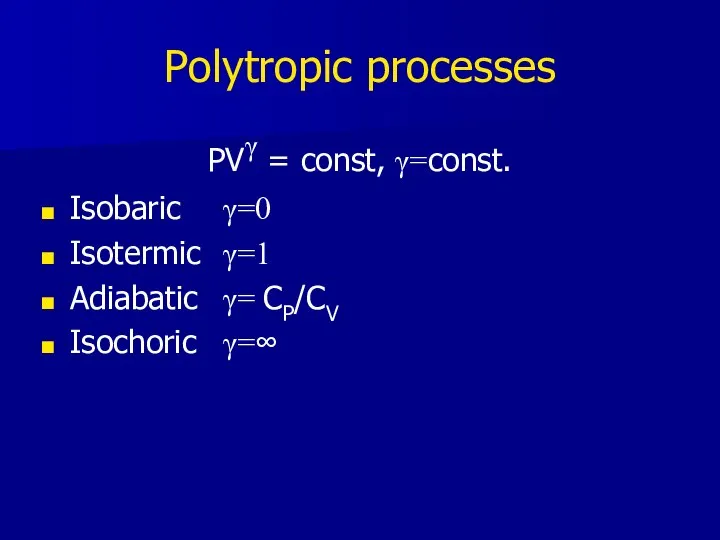
- 21. Polytropic processes PVγ = const, γ=const. Isobaric γ=0 Isotermic γ=1 Adiabatic γ= CP/CV Isochoric γ=∞
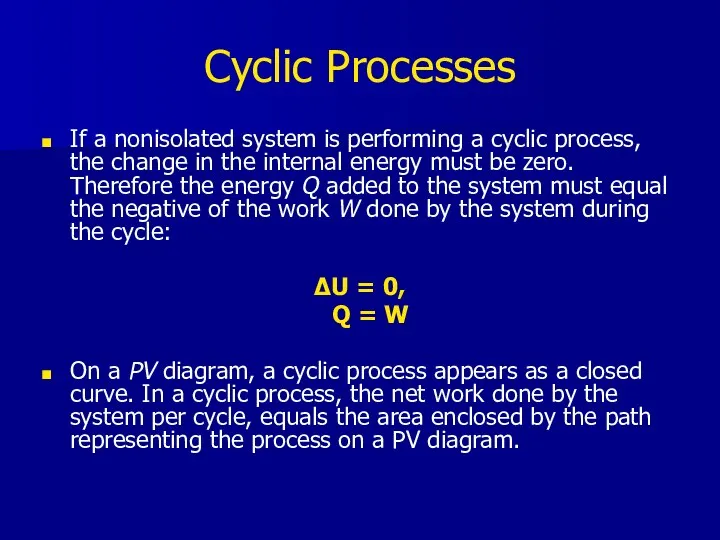
- 22. Cyclic Processes If a nonisolated system is performing a cyclic process, the change in the internal
- 24. Скачать презентацию







 Siltumfizikas pamati. Enerģijas mērvienības, spiediens, degšana, siltumapmaiņa, tvaika veidošanās
Siltumfizikas pamati. Enerģijas mērvienības, spiediens, degšana, siltumapmaiņa, tvaika veidošanās Віктар Анатольевіч Шніп
Віктар Анатольевіч Шніп Работа силы тяжести
Работа силы тяжести Физические свойства воды в разных агрегатных состояниях
Физические свойства воды в разных агрегатных состояниях Энергия движущейся воды и ветра
Энергия движущейся воды и ветра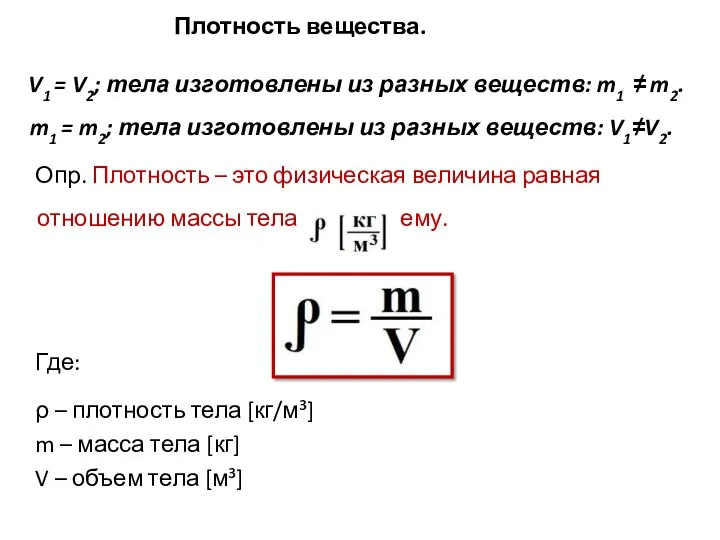 Плотность вещества. V1 = V2; тела изготовлены из разных веществ: m1 ≠ m2. m1 = m2; тела изготовлены из разных веществ: V1≠V2. Опр. Плотность – это физическая величина равная отношению массы тела к его объему. Где: ρ – плотность тела [кг/м³] m
Плотность вещества. V1 = V2; тела изготовлены из разных веществ: m1 ≠ m2. m1 = m2; тела изготовлены из разных веществ: V1≠V2. Опр. Плотность – это физическая величина равная отношению массы тела к его объему. Где: ρ – плотность тела [кг/м³] m Исследование радиального профиля параметров активной среды лазеров с разрядом в полом катоде
Исследование радиального профиля параметров активной среды лазеров с разрядом в полом катоде Ньютон. Совершенны ли законы Ньютона?
Ньютон. Совершенны ли законы Ньютона? Направление индукционного тока. Правило Ленца. Явление самоиндукции
Направление индукционного тока. Правило Ленца. Явление самоиндукции Освітньо-професійна програма. Середня освіта (фізика)
Освітньо-професійна програма. Середня освіта (фізика) Загальні відомості про рух
Загальні відомості про рух  製造廠家及資訊系統之更新與維護 高壓用電設備試驗作業要點 說明會
製造廠家及資訊系統之更新與維護 高壓用電設備試驗作業要點 說明會 Тиристор. Общая характеристика оптоэлектронных приборов
Тиристор. Общая характеристика оптоэлектронных приборов Аттестационная работа. Методическая разработка урока по теме «Энергосбережение». (8 класс)
Аттестационная работа. Методическая разработка урока по теме «Энергосбережение». (8 класс) Ядерная физика
Ядерная физика ХИРОСИМА И НАГАСАКИ Презентация по физике ученицы 11 класса А Севастьяновой Ксении
ХИРОСИМА И НАГАСАКИ Презентация по физике ученицы 11 класса А Севастьяновой Ксении Варианты самостоятельной работы
Варианты самостоятельной работы Валы и оси
Валы и оси Продление ресурса и безопасность АЭС
Продление ресурса и безопасность АЭС Сопряжение
Сопряжение Теория волновых процессов
Теория волновых процессов Открытие и исследование векторных бозонов в эксперименте ATLAS
Открытие и исследование векторных бозонов в эксперименте ATLAS Система питания дизеля
Система питания дизеля Динамика вращательного движения
Динамика вращательного движения Практическая физика
Практическая физика Математический маятник
Математический маятник Условия полного внутреннего отражения
Условия полного внутреннего отражения Презентация по физике Молекулярная физика и термодинамика
Презентация по физике Молекулярная физика и термодинамика