Содержание
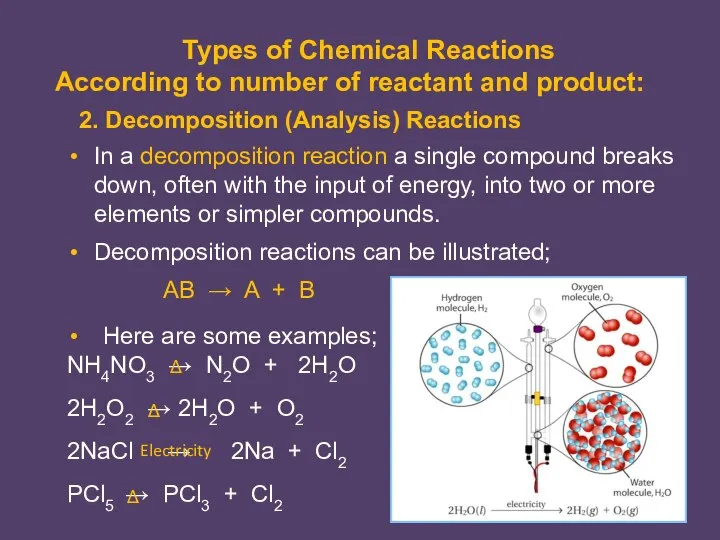
- 2. In a decomposition reaction a single compound breaks down, often with the input of energy, into
- 3. In a displacement reaction a single element reacts with a compound and displaces another element from
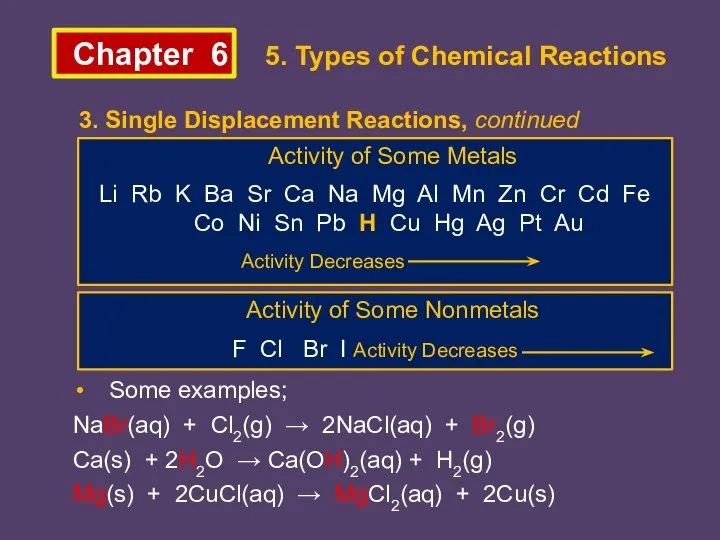
- 4. Chapter 6 3. Single Displacement Reactions, continued 5. Types of Chemical Reactions Some examples; NaBr(aq) +
- 5. In a double-displacement reaction two compounds in aqueous solution appear to exchange ions and form two
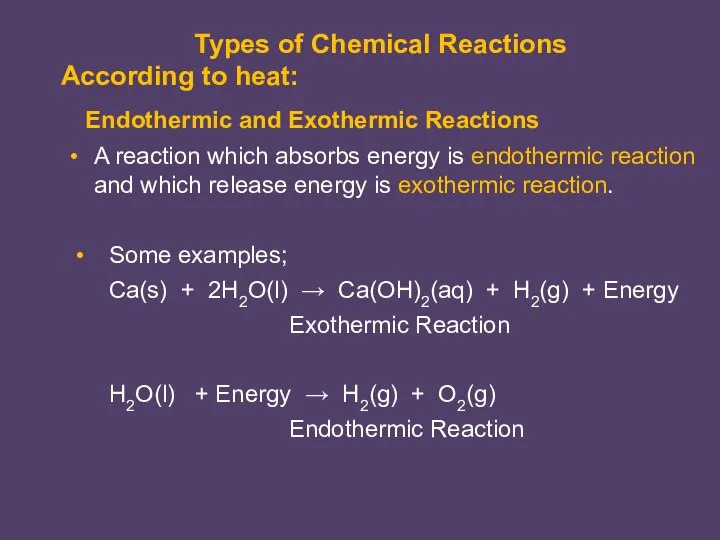
- 6. A reaction which absorbs energy is endothermic reaction and which release energy is exothermic reaction. Endothermic
- 7. Types of Chemical Reaction According to states of substance: Homogenous reactions:Reactants and products are in same
- 9. Скачать презентацию


 Насыщенные углеводороды
Насыщенные углеводороды Нәруыз. Қасиеті және қызметі
Нәруыз. Қасиеті және қызметі Основы теории смазывания и стандарты качества моторных масел
Основы теории смазывания и стандарты качества моторных масел Химические источники тока
Химические источники тока Живые системы для производства углеводородов
Живые системы для производства углеводородов Презентация ОБЩАЯ ЖЕСТКОСТЬ ВОДЫ
Презентация ОБЩАЯ ЖЕСТКОСТЬ ВОДЫ  Массасы 4,2 г көміртек (IV) оксиді сумен әрекеттескенде қанша грамм көмір қышқылы (Н2СО3) түзілетінін есепте
Массасы 4,2 г көміртек (IV) оксиді сумен әрекеттескенде қанша грамм көмір қышқылы (Н2СО3) түзілетінін есепте Нафта
Нафта  Point defects. Line defects. Surface Imperfections
Point defects. Line defects. Surface Imperfections Кристаллохимия как наука
Кристаллохимия как наука Кыргызстандагы химия онор жайы
Кыргызстандагы химия онор жайы Химическая связь. Природа химической связи
Химическая связь. Природа химической связи Nucleic acids
Nucleic acids Электролиз воды
Электролиз воды «Голубое золото» 10 класс базовый уровень
«Голубое золото» 10 класс базовый уровень  Схема катаболизма углеродного скилета аминокислот
Схема катаболизма углеродного скилета аминокислот Глицерин
Глицерин Природные источники углеводородов и их переработка
Природные источники углеводородов и их переработка Введение в биохимию. Строение и функции белков (часть 1)
Введение в биохимию. Строение и функции белков (часть 1) Химия и сельское хозяйство
Химия и сельское хозяйство Физические свойства металлов. Сплавы
Физические свойства металлов. Сплавы Презентация по химии Электролитическая диссоциация
Презентация по химии Электролитическая диссоциация  Հիալուրոնաթթվի անջատումը ձվի կեղևից և դրա նույնականացումը բարձրարդյունավետ. Հեղուկային քրոմատոգրաֆիայի եղանակով
Հիալուրոնաթթվի անջատումը ձվի կեղևից և դրա նույնականացումը բարձրարդյունավետ. Հեղուկային քրոմատոգրաֆիայի եղանակով Стеклянные товары. Основные сырьевые материалы для производства стекла
Стеклянные товары. Основные сырьевые материалы для производства стекла Презентация по Химии "Характеристика химического элемента – неметалла на основании его положения в Периодической системе хими
Презентация по Химии "Характеристика химического элемента – неметалла на основании его положения в Периодической системе хими Может ли вода приносить вред здоровью? Авторы: Козлова Ирина Владимировна, Ушакова Кристина Николаевна, 9 класс МОУ «СОШ №12», г. А
Может ли вода приносить вред здоровью? Авторы: Козлова Ирина Владимировна, Ушакова Кристина Николаевна, 9 класс МОУ «СОШ №12», г. А Строение полимера. Мономерное звено. Урок № 27
Строение полимера. Мономерное звено. Урок № 27 Ксенобиотики. Микросомальное окисление
Ксенобиотики. Микросомальное окисление