Содержание
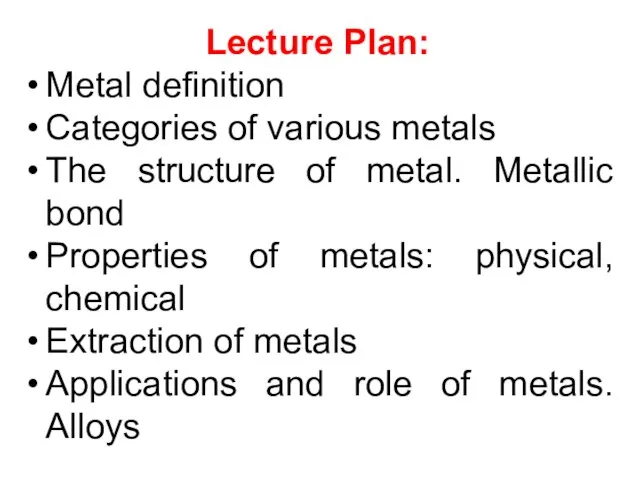
- 2. Lecture Plan: Metal definition Categories of various metals The structure of metal. Metallic bond Properties of
- 3. OBJECTIVES: Understand the physical properties of metals. Explains the chemical properties of metals. Explain how the
- 4. A metal (from Greek μέταλλον métallon, "mine, quarry, metal") is a material (an element, compound, or
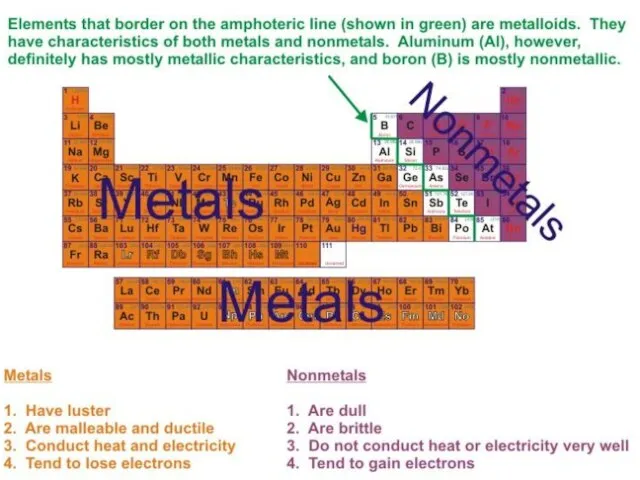
- 6. Metallic Elements: Alkali metals (group IA): Li, Na, K, Rb, Cs, Fr Alkali earth metals (group
- 7. Metallic Elements: 4) Post-transition metals: Al, In, Ga, Sn, Tl, Pb, Bi, Po 5) Lanthanides 6)
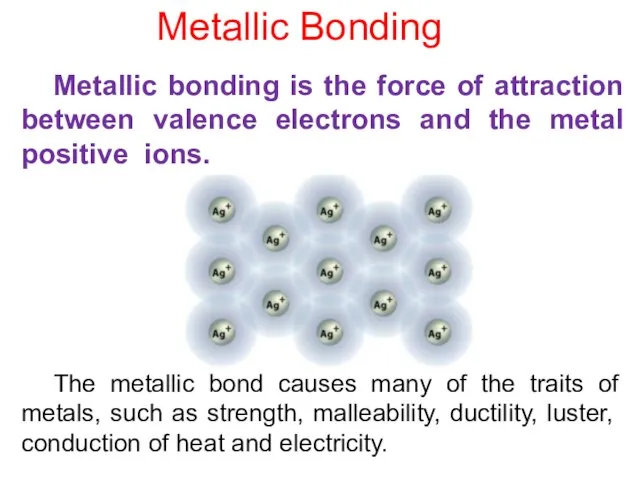
- 8. The metallic bond causes many of the traits of metals, such as strength, malleability, ductility, luster,
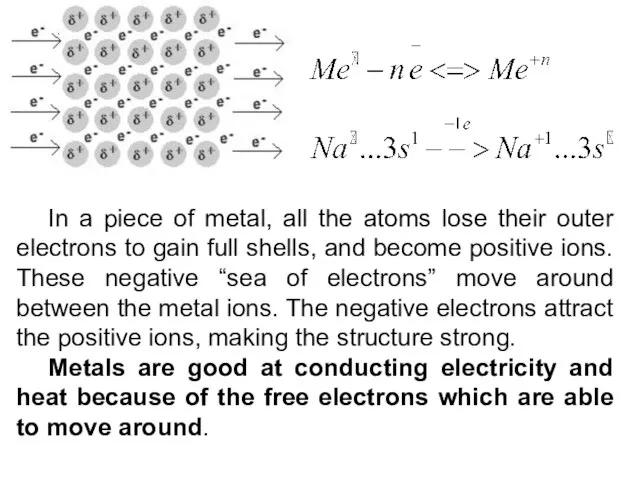
- 9. In a piece of metal, all the atoms lose their outer electrons to gain full shells,
- 10. REASONS: GIVE 3 REASONS WHY TUNGSTEN IS USED TO MAKE THE FILAMENT INSIDE AN ELECTRIC BULB?
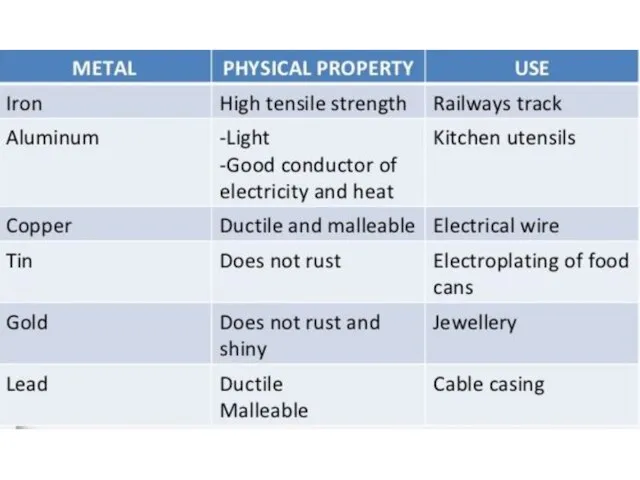
- 11. PHYSICAL PROPERTIES OF METALS Good electrical and heat conductors. Malleable - can be beaten into thin
- 12. Density of Metals Light metals Heavy metals Magnesium 1,74 g/cm3 Lead 11,3 g/cm3 Gold 19,3 g/cm3
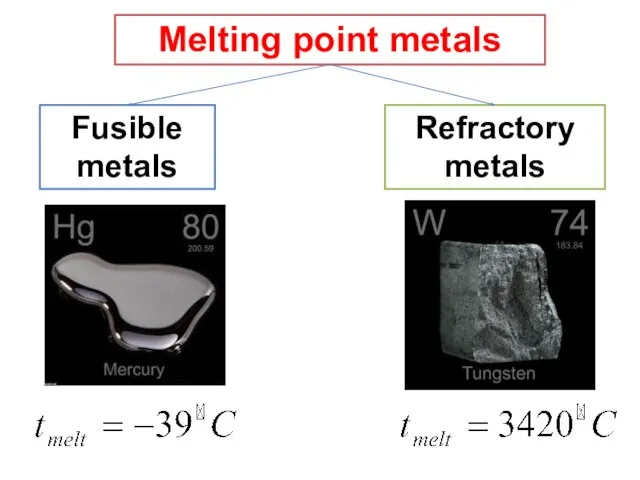
- 13. Melting point metals Refractory metals Fusible metals
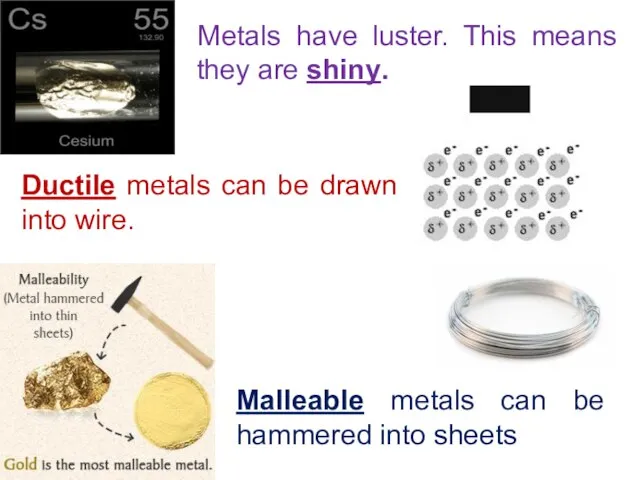
- 14. Metals have luster. This means they are shiny. Ductile metals can be drawn into wire. Malleable
- 15. A chemical property of metal is its reaction with water and oxygen. This results in corrosion
- 16. These properties make metals most useful in daily life.
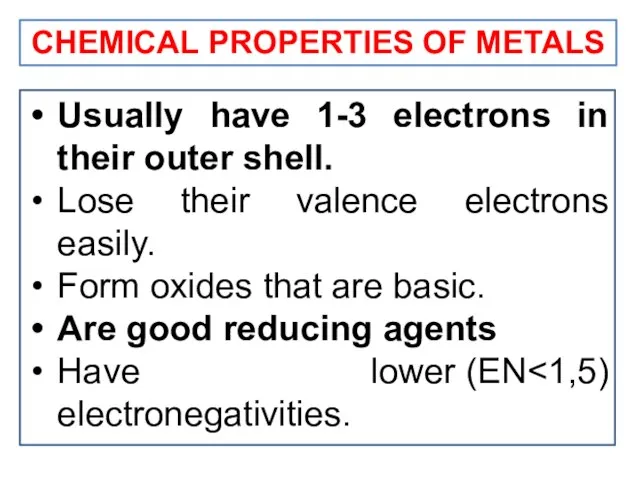
- 18. CHEMICAL PROPERTIES OF METALS Usually have 1-3 electrons in their outer shell. Lose their valence electrons
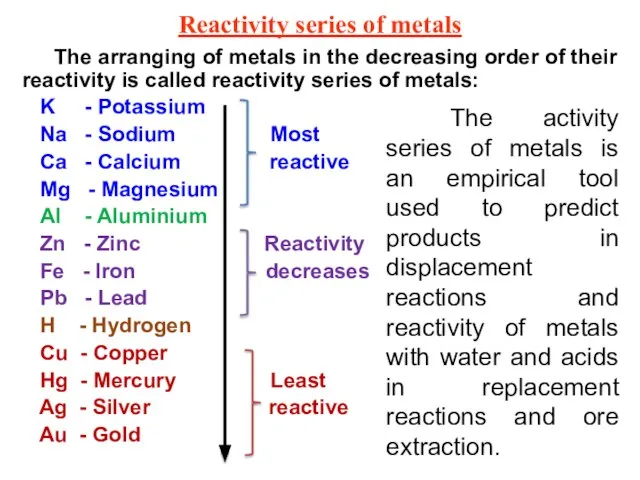
- 19. Reactivity series of metals The arranging of metals in the decreasing order of their reactivity is
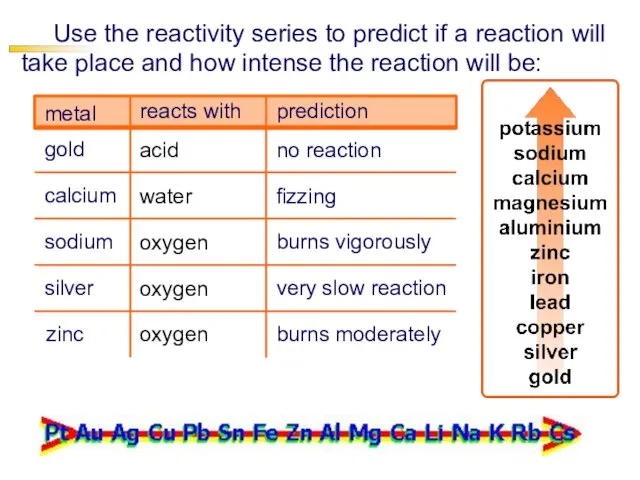
- 20. Use the reactivity series to predict if a reaction will take place and how intense the
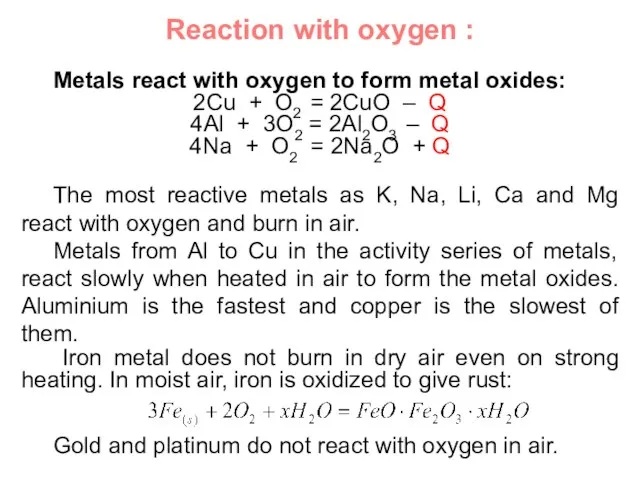
- 21. Reaction with oxygen : Metals react with oxygen to form metal oxides: 2Cu + O2 =
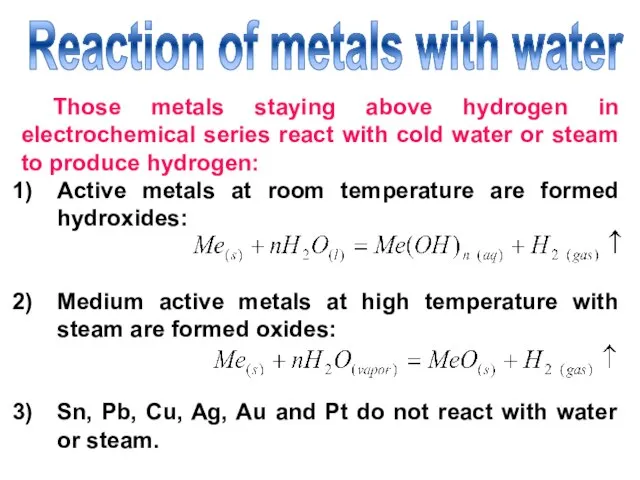
- 22. Reaction of metals with water Those metals staying above hydrogen in electrochemical series react with cold
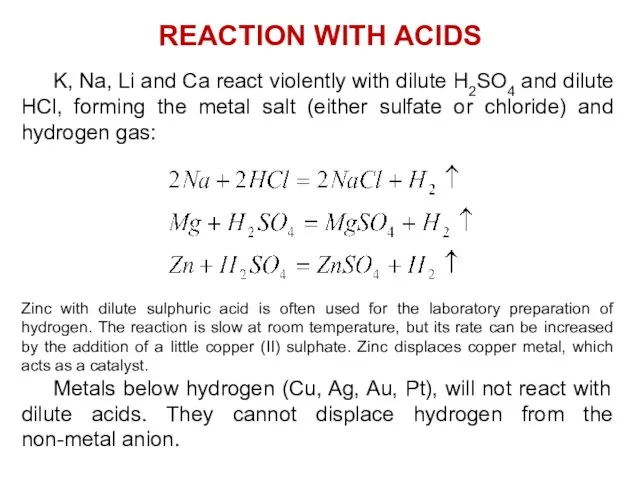
- 23. K, Na, Li and Ca react violently with dilute H2SO4 and dilute HCl, forming the metal
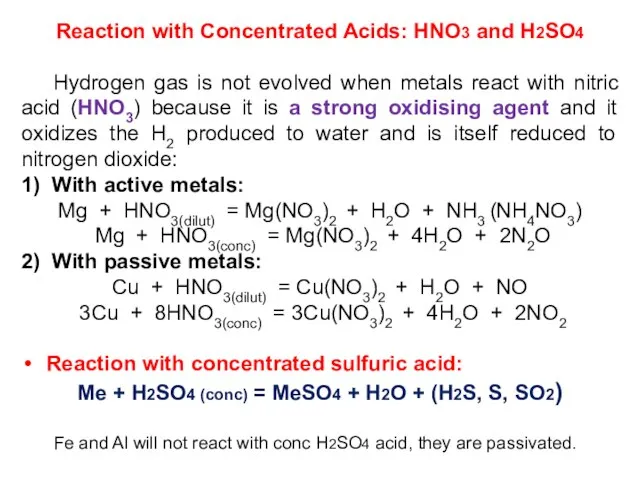
- 24. Reaction with Concentrated Acids: HNO3 and H2SO4 Hydrogen gas is not evolved when metals react with
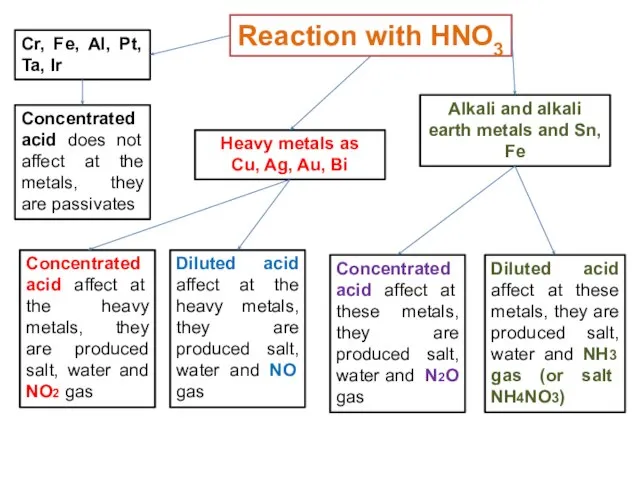
- 25. Reaction with HNO3 Concentrated acid does not affect at the metals, they are passivates Cr, Fe,
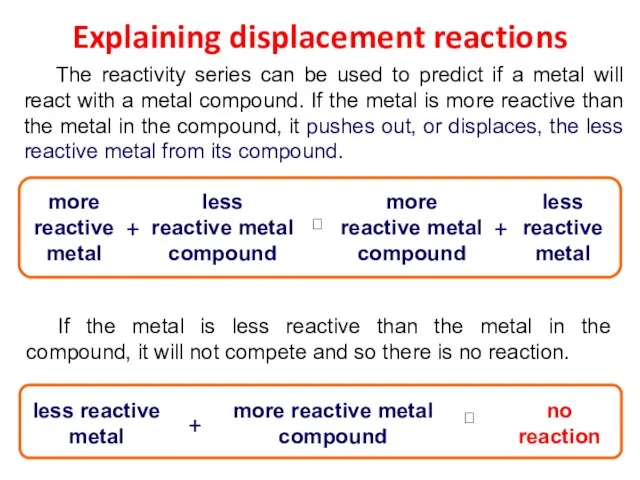
- 26. Explaining displacement reactions The reactivity series can be used to predict if a metal will react
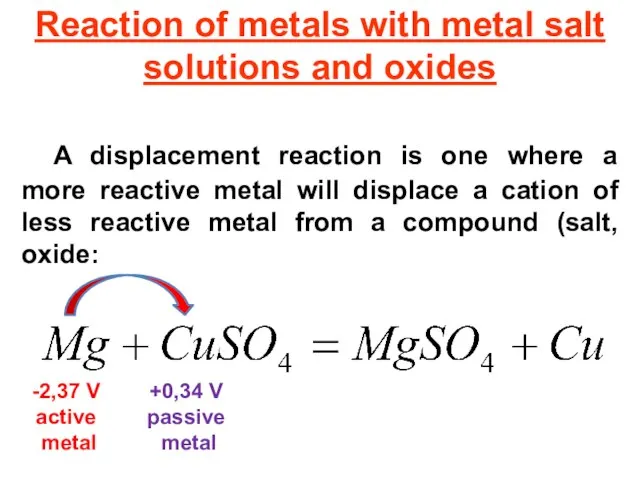
- 27. Reaction of metals with metal salt solutions and oxides A displacement reaction is one where a
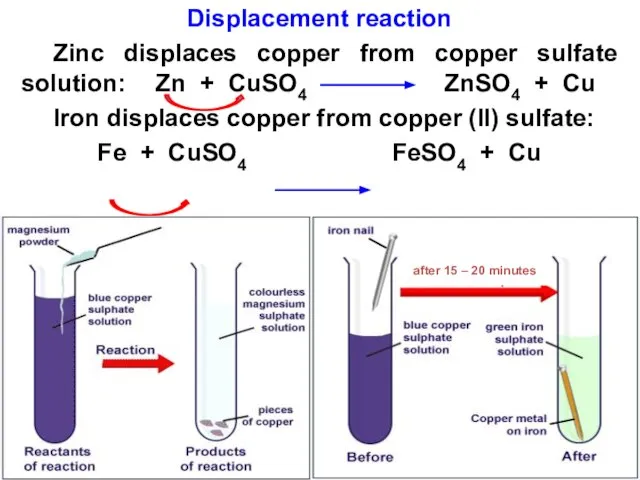
- 28. Displacement reaction Zinc displaces copper from copper sulfate solution: Zn + CuSO4 ZnSO4 + Cu Iron
- 29. The more reactive aluminium takes the oxygen from the less reactive iron. The reaction gets so
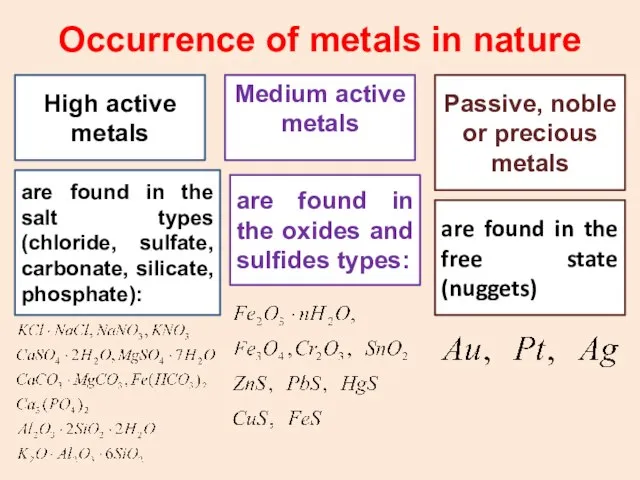
- 30. Occurrence of metals : Some metals like gold, silver, platinum etc are found in the free
- 31. Occurrence of metals in nature High active metals Medium active metals Passive, noble or precious metals
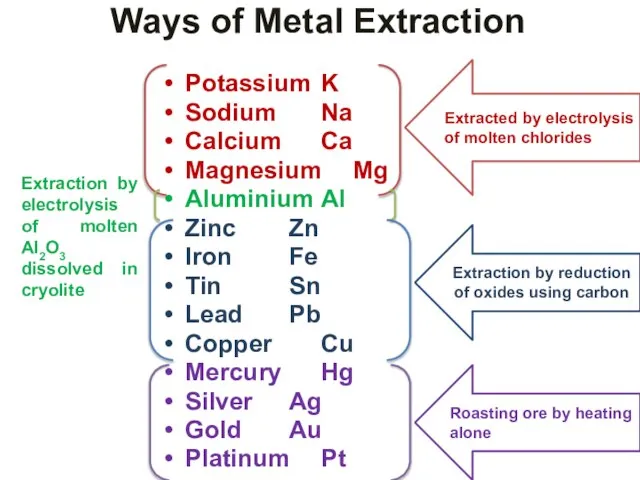
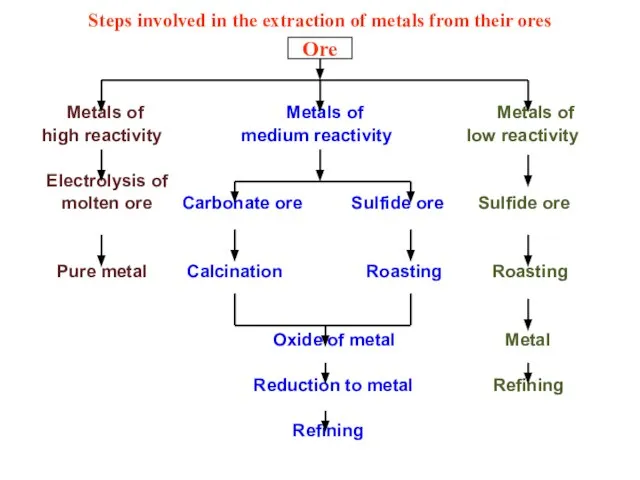
- 32. Extraction of metals from their ores : The various processes involved in the extraction of metals
- 33. Ways of Metal Extraction Potassium K Sodium Na Calcium Ca Magnesium Mg Aluminium Al Zinc Zn
- 34. Steps involved in the extraction of metals from their ores Metals of Metals of Metals of
- 35. Using of Metals Metal used in manufacturing are usually alloys, which are composed of two or
- 36. Ferrous Metals (black): Ferrous metals are based on iron: the group includes steel and cast iron.
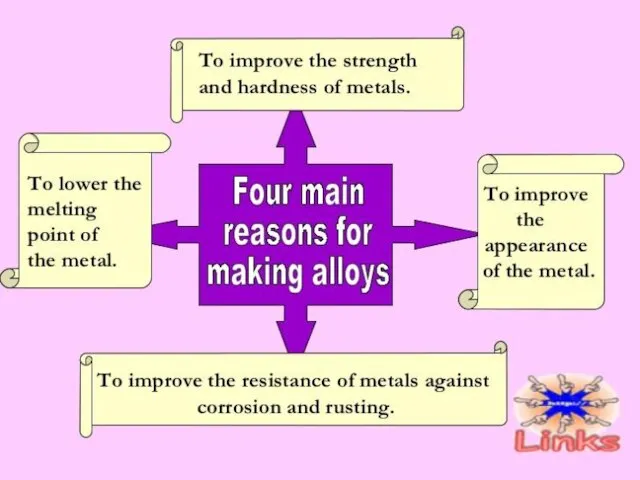
- 37. METALS ALLOYS An alloy is a homogeneous mixture of a metal with other metals or non
- 41. Скачать презентацию
















 Метаболизм липидов. Характеристика липидов. Значение. Представители. Эйкозаноиды. (Лекция 1-2)
Метаболизм липидов. Характеристика липидов. Значение. Представители. Эйкозаноиды. (Лекция 1-2) Бинарный урок - конференция по физике и химии
Бинарный урок - конференция по физике и химии Біохімія білків
Біохімія білків КАЛЬЦИЙ Ca
КАЛЬЦИЙ Ca  Презентация по Химии "Угольная кислота и ее соли" - скачать смотреть
Презентация по Химии "Угольная кислота и ее соли" - скачать смотреть  Эластомеры (резины)
Эластомеры (резины) Свойства и способы получения аминокислот. Их значение и применение.
Свойства и способы получения аминокислот. Их значение и применение. Введение в токсикологическую химию
Введение в токсикологическую химию Трансформация соединений азота
Трансформация соединений азота Стерилизация технологических потоков и оборудования
Стерилизация технологических потоков и оборудования Жувальна гумка Виконав учень 11-Б Надворний Ярослав
Жувальна гумка Виконав учень 11-Б Надворний Ярослав  Синтез карбамида (лекция 17)
Синтез карбамида (лекция 17) Задача №5. Аккумулятор на основе железа
Задача №5. Аккумулятор на основе железа Применение интерактивной доски на уроках химии Учитель химии МОУ «СОШ п. Первомайский» Кириченко Е.Н.
Применение интерактивной доски на уроках химии Учитель химии МОУ «СОШ п. Первомайский» Кириченко Е.Н. Презентация Жидкие кристаллы
Презентация Жидкие кристаллы  Вміст нітратів в продуктах харчування
Вміст нітратів в продуктах харчування Характеристика дефектов. Растворы влияющие на степень износа тканей
Характеристика дефектов. Растворы влияющие на степень износа тканей Исследование интерактивной компьютерной химической модели
Исследование интерактивной компьютерной химической модели “Необроблений” мас-спектр (йонізація електронним ударом)
“Необроблений” мас-спектр (йонізація електронним ударом)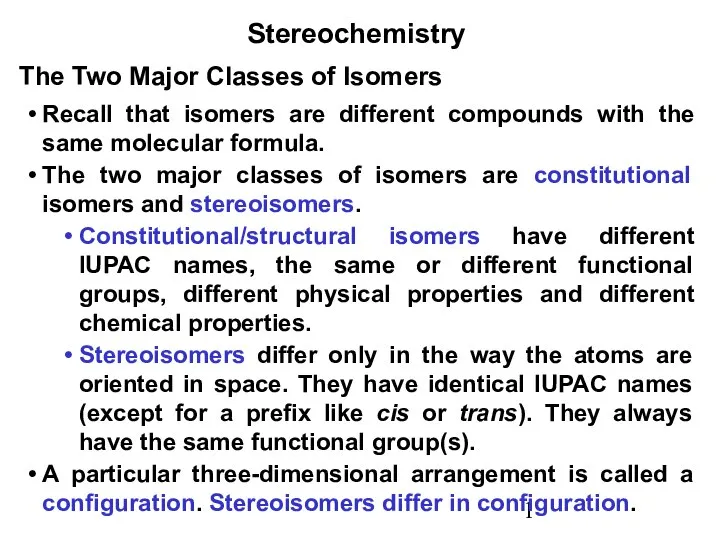 Stereochemistry.Isomers are different compounds
Stereochemistry.Isomers are different compounds Гомополисахариды (углеводы растений)
Гомополисахариды (углеводы растений) Использование кинетики в фармации
Использование кинетики в фармации Круговорот азота в природе
Круговорот азота в природе ОСНОВНЫЕ ПОНЯТИЯ ХИМИИ 1 полугодие 8 класс Л.И.Настина, учитель химии
ОСНОВНЫЕ ПОНЯТИЯ ХИМИИ 1 полугодие 8 класс Л.И.Настина, учитель химии  Сульфиды. Лекция 8
Сульфиды. Лекция 8 Химический состав клетки. Вода и ее роль в жизнедеятельности клетки. Минеральные вещества
Химический состав клетки. Вода и ее роль в жизнедеятельности клетки. Минеральные вещества Рибозимы. Строение
Рибозимы. Строение Вода: смерть чи життя? Вивчення якості води у водоймах і водоспадах.
Вода: смерть чи життя? Вивчення якості води у водоймах і водоспадах.