Содержание
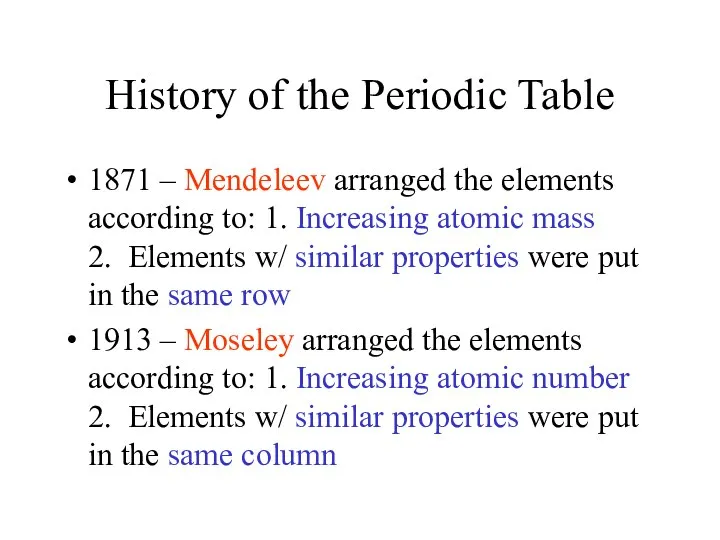
- 2. History of the Periodic Table 1871 – Mendeleev arranged the elements according to: 1. Increasing atomic
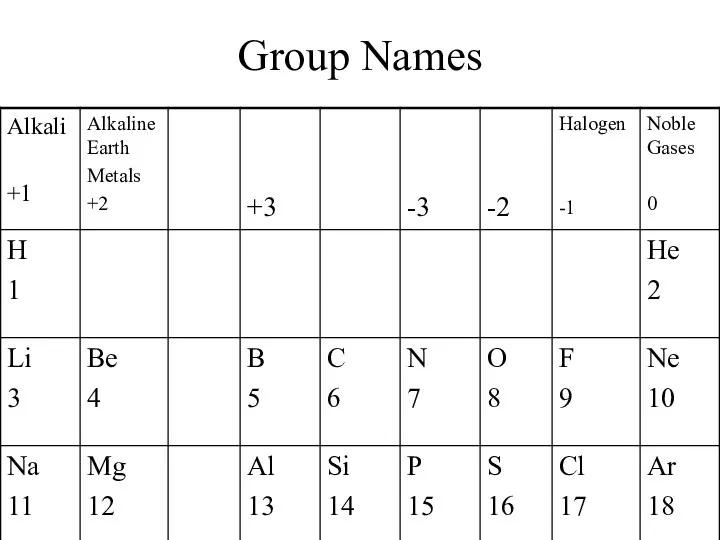
- 3. Group Names
- 4. S & P block – Representative Elements Metalloids (Semimetals, Semiconductors) – B,Si, Ge, As, Sb, Te
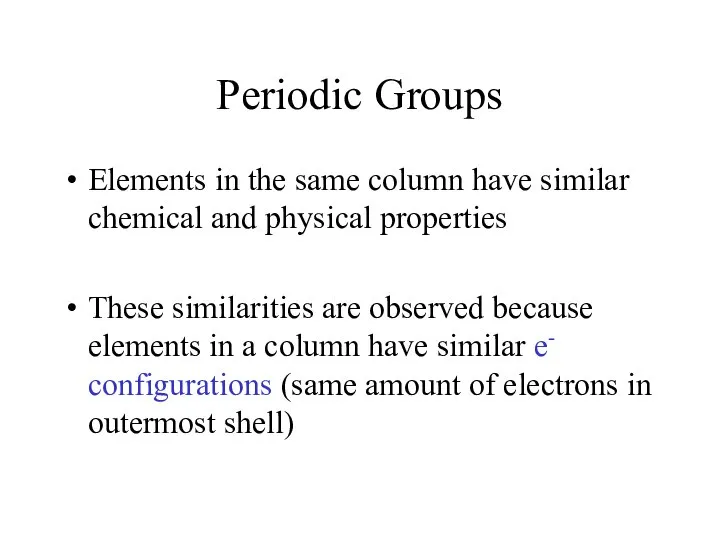
- 5. Periodic Groups Elements in the same column have similar chemical and physical properties These similarities are
- 6. Periodic Trends Periodic Trends – patterns (don’t always hold true) can be seen with our current
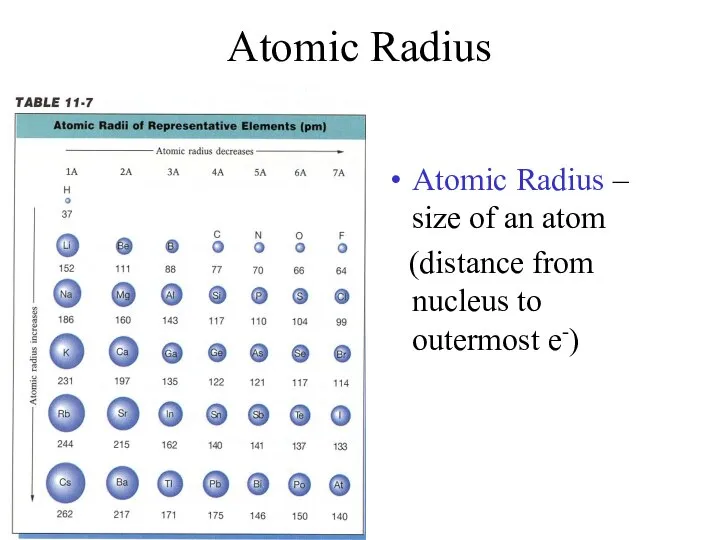
- 7. Atomic Radius Atomic Radius – size of an atom (distance from nucleus to outermost e-)
- 8. Atomic Radius Trend Group Trend – As you go down a column, atomic radius increases As
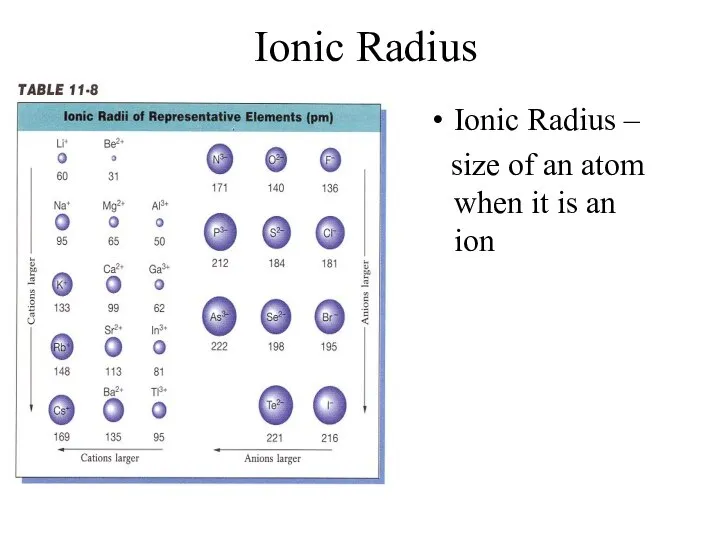
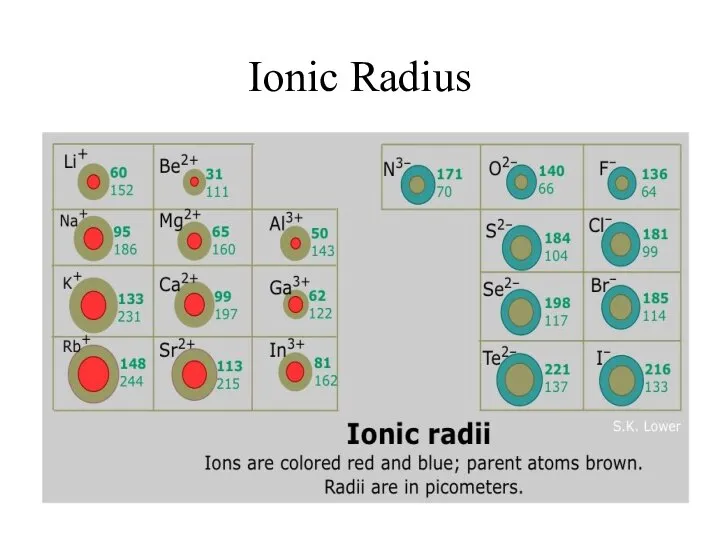
- 9. Ionic Radius Ionic Radius – size of an atom when it is an ion
- 10. Ionic Radius Trend Metals – lose e-, which means more p+ than e- (more attraction) SO…
- 11. Ionic Radius Trend Group Trend – As you go down a column, ionic radius increases Periodic
- 12. Ionic Radius
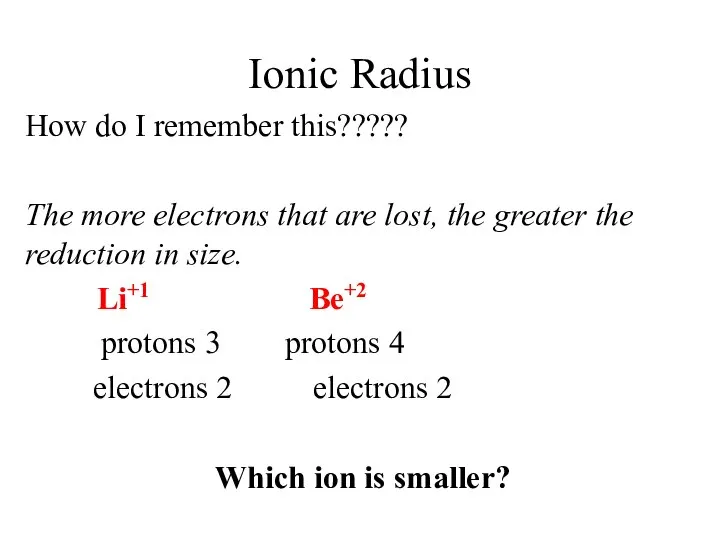
- 13. Ionic Radius How do I remember this????? The more electrons that are lost, the greater the
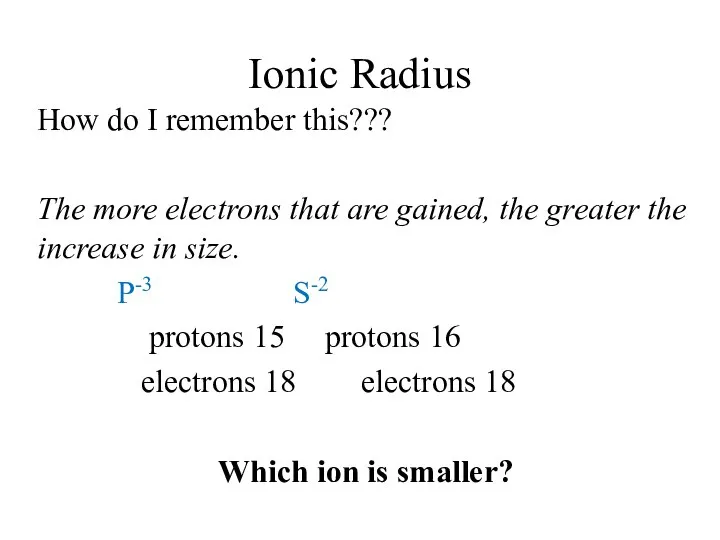
- 14. Ionic Radius How do I remember this??? The more electrons that are gained, the greater the
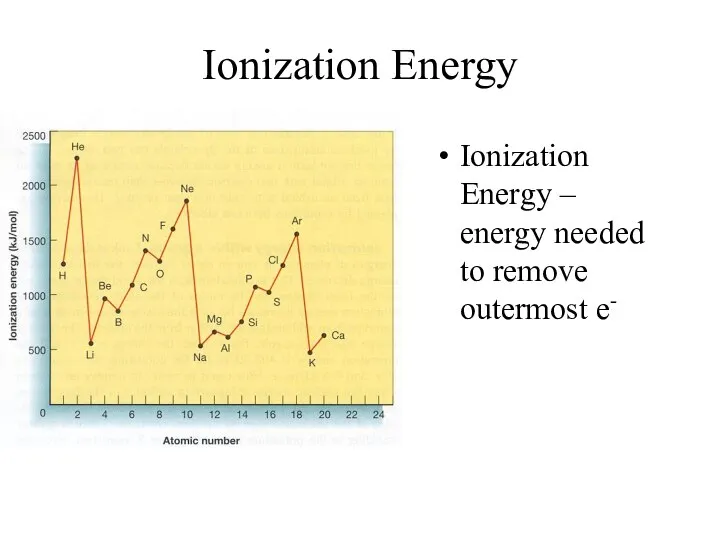
- 15. Ionization Energy Ionization Energy – energy needed to remove outermost e-
- 16. Ionization Energy Group Trend – As you go down a column, ionization energy decreases As you
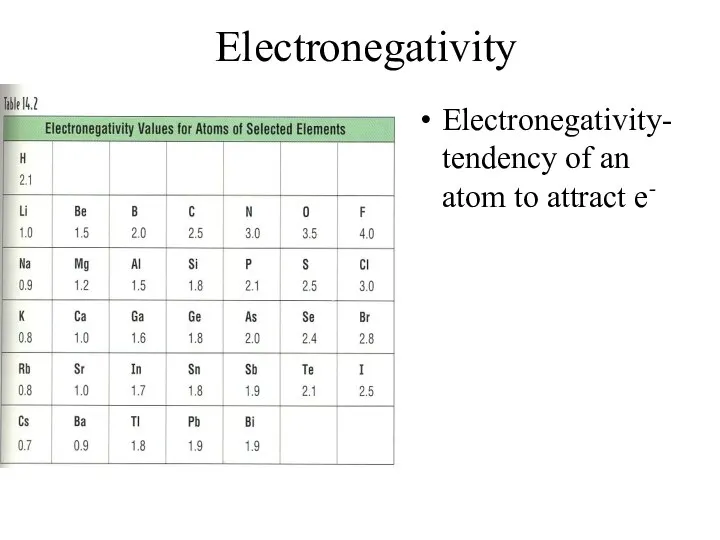
- 17. Electronegativity Electronegativity- tendency of an atom to attract e-
- 18. Electronegativity Trend Group Trend – As you go down a column, electronegativity decreases As you go
- 19. Reactivity Reactivity – tendency of an atom to react Metals – lose e- when they react,
- 21. Скачать презентацию







 Chemistry
Chemistry Получение кормовой добавки на основе выжимок томатов и рисовой мучки
Получение кормовой добавки на основе выжимок томатов и рисовой мучки Липиды. Общая характеристика липидов
Липиды. Общая характеристика липидов Органическая химия- химия соединений углерода
Органическая химия- химия соединений углерода Презентация по Химии "Гетероциклические соединения" - скачать смотреть
Презентация по Химии "Гетероциклические соединения" - скачать смотреть  Воздух. Постоянные компоненты воздуха
Воздух. Постоянные компоненты воздуха Безопасное использование атразина, как гербицида в сельском хозяйстве
Безопасное использование атразина, как гербицида в сельском хозяйстве Решение расчётных задач
Решение расчётных задач Виды присадок к моторным топливам. Присадки к дизельному топливу
Виды присадок к моторным топливам. Присадки к дизельному топливу Композитные материалы
Композитные материалы Элементы теории горения
Элементы теории горения Презентация к открытому уроку Учитель химии МОУ «Гимназия №1» г. Саратов Шишкина И.Ю.
Презентация к открытому уроку Учитель химии МОУ «Гимназия №1» г. Саратов Шишкина И.Ю.  Оксид азота(2) NO
Оксид азота(2) NO Введение в минералогию. Лекция 1
Введение в минералогию. Лекция 1 Основания, кислоты, соли Комбинированный урок химии в 8 классе
Основания, кислоты, соли Комбинированный урок химии в 8 классе Тяжелые металлы. Опасность токсичных металлов для организма
Тяжелые металлы. Опасность токсичных металлов для организма Көміртек жене оның қосылыстары
Көміртек жене оның қосылыстары Алканы. Пропан - С3Н8
Алканы. Пропан - С3Н8 Положение металлов в ПСХЭ Д.И. Менделеева. Общие физические свойства металлов
Положение металлов в ПСХЭ Д.И. Менделеева. Общие физические свойства металлов Кислоты
Кислоты Типы химических реакций в органической химии. Типы реакций в органике
Типы химических реакций в органической химии. Типы реакций в органике Презентация по Химии "Химические свойства металлов. Электрохимический ряд напряжения металлов. 11-й класс" - скачать смотреть
Презентация по Химии "Химические свойства металлов. Электрохимический ряд напряжения металлов. 11-й класс" - скачать смотреть  Основи процесів горіння. Полум’я. Процеси, що відбуваються у полум’ї. (Розділ 1.1.2)
Основи процесів горіння. Полум’я. Процеси, що відбуваються у полум’ї. (Розділ 1.1.2) Пластмаси.Синтетичні каучуки.Гума виконала учениця 11-Ф класу Бабич Роксолана
Пластмаси.Синтетичні каучуки.Гума виконала учениця 11-Ф класу Бабич Роксолана  G11. Химический состав
G11. Химический состав Презентация по Химии "ПОЛУЧЕНИЕ И СВОЙСТВА СТАБИЛЬНЫХ ИЗОТОПОВ КРЕМНИЯ ВЫСОКОЙ ХИМИЧЕСКОЙ И ИЗОТОПНОЙ ЧИСТОТЫ" - скачать смо
Презентация по Химии "ПОЛУЧЕНИЕ И СВОЙСТВА СТАБИЛЬНЫХ ИЗОТОПОВ КРЕМНИЯ ВЫСОКОЙ ХИМИЧЕСКОЙ И ИЗОТОПНОЙ ЧИСТОТЫ" - скачать смо МЕТАЛЛЫ В ПЕРИОДИЧЕСКОЙ СИСТЕМЕ ХИМИЧЕСКИХ ЭЛЕМЕНТОВ Д.И. МЕНДЕЛЕЕВА Петреня Игорь Михайлович, учитель химии и биологии госуд
МЕТАЛЛЫ В ПЕРИОДИЧЕСКОЙ СИСТЕМЕ ХИМИЧЕСКИХ ЭЛЕМЕНТОВ Д.И. МЕНДЕЛЕЕВА Петреня Игорь Михайлович, учитель химии и биологии госуд Биополимеры. Классификация полисахаридов (гликаны)
Биополимеры. Классификация полисахаридов (гликаны)