Содержание
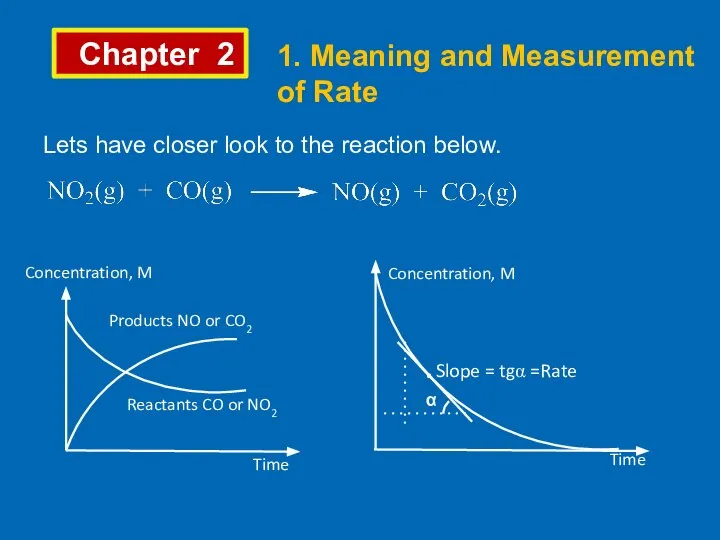
- 2. Chapter 2 1. Meaning and Measurement of Rate Lets have closer look to the reaction below.
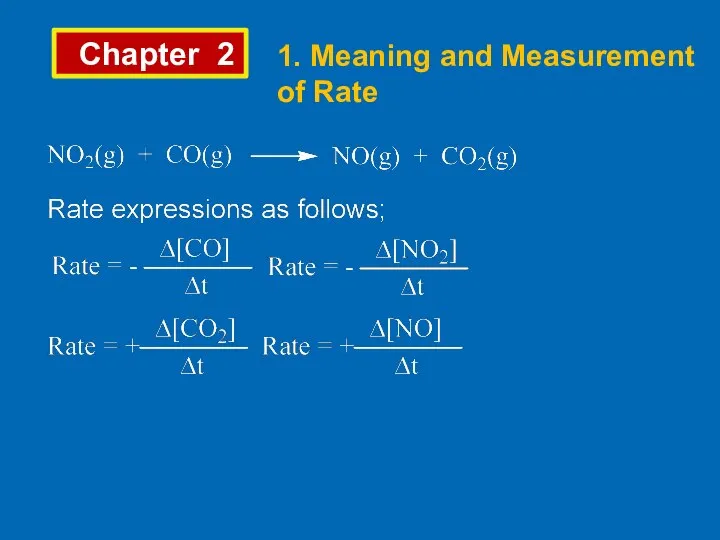
- 3. Chapter 2 1. Meaning and Measurement of Rate
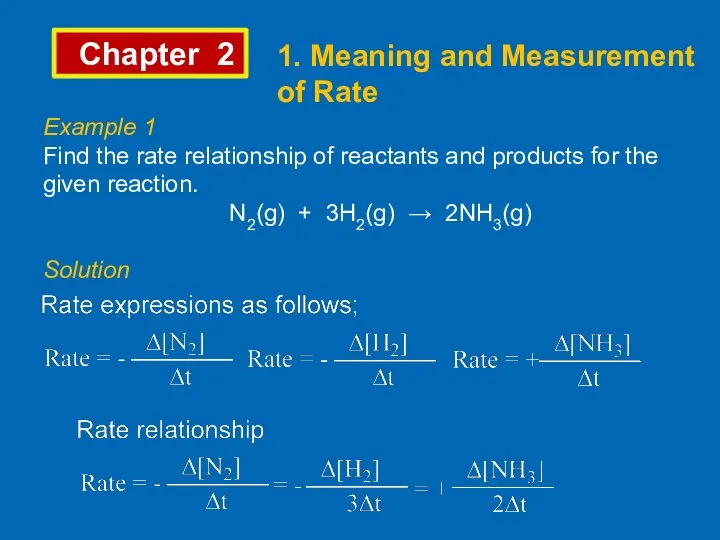
- 4. Chapter 2 1. Meaning and Measurement of Rate Example 1 Find the rate relationship of reactants
- 5. The decomposition of dinitrogen pentoxide can be represented by the equation; 2N2O5 → 4NO2 + O2
- 6. RateN2O5 = (0.008 – 0.004)/20 = 0.0002 = 2.10−4 mol/L. s
- 7. Chapter 2 1. Meaning and Measurement of Rate Example 2 In the following decomposition reaction, 2N2O5
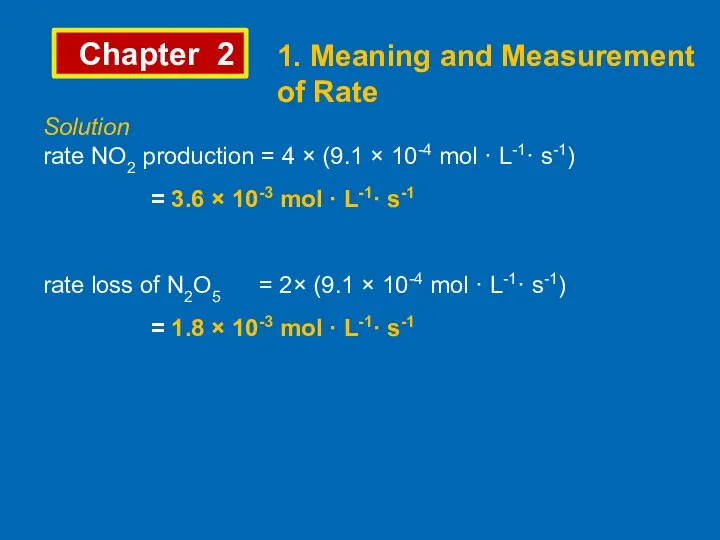
- 8. Chapter 2 1. Meaning and Measurement of Rate Solution rate NO2 production = 4 × (9.1
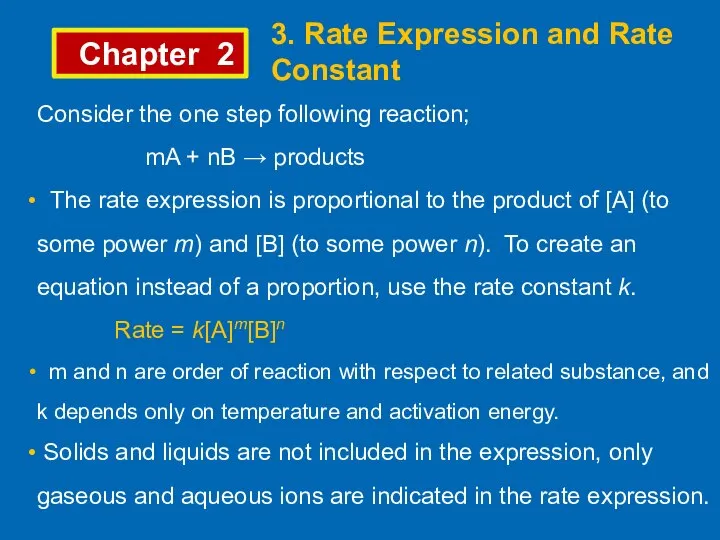
- 9. Chapter 2 3. Rate Expression and Rate Constant Consider the one step following reaction; mA +
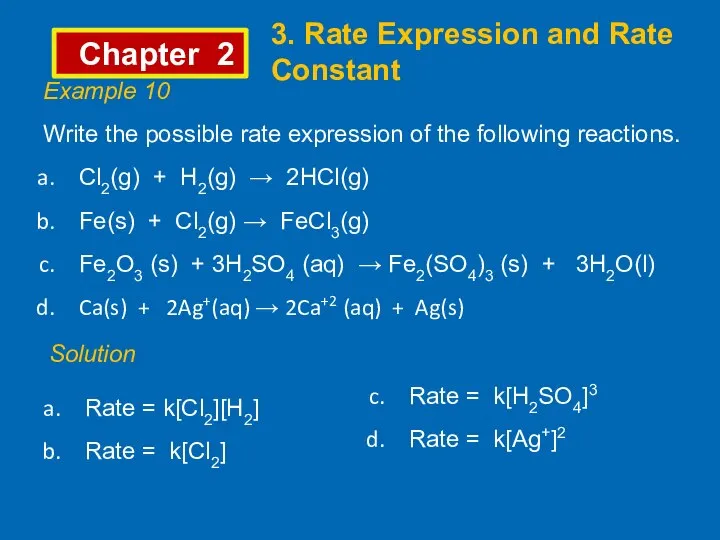
- 10. Chapter 2 3. Rate Expression and Rate Constant Example 10 Write the possible rate expression of
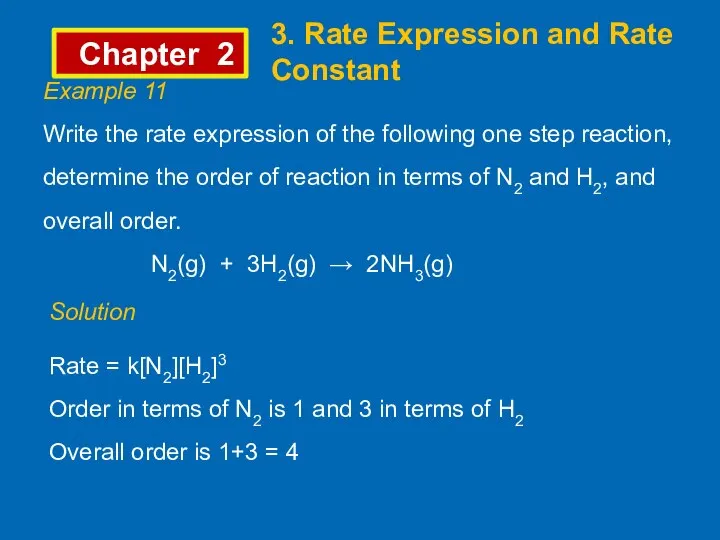
- 11. Chapter 2 3. Rate Expression and Rate Constant Example 11 Write the rate expression of the
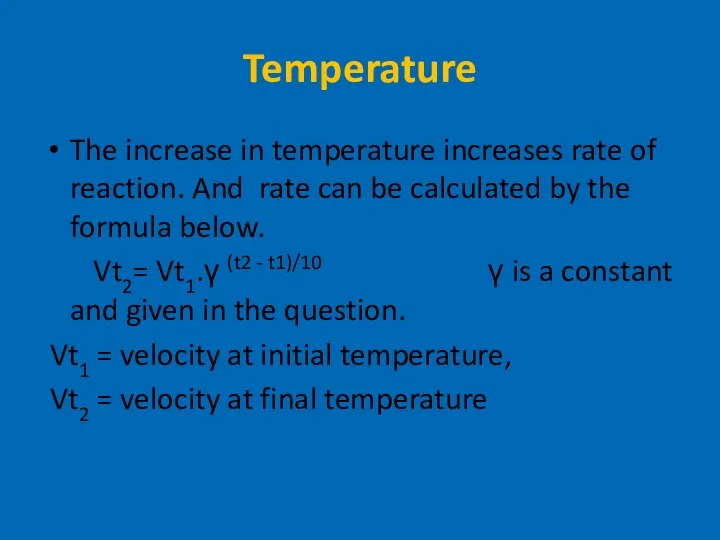
- 12. Temperature The increase in temperature increases rate of reaction. And rate can be calculated by the
- 14. Скачать презентацию



 Сапонины. Строение сапонинов
Сапонины. Строение сапонинов Хроматографический анализ
Хроматографический анализ Аттестационная работа. Образовательная программа внеурочной деятельности по химии Занимательная химия
Аттестационная работа. Образовательная программа внеурочной деятельности по химии Занимательная химия Ксенобиотики в окружающей среде и живых организмах. (Лекция 2)
Ксенобиотики в окружающей среде и живых организмах. (Лекция 2) Химические свойства алкинов
Химические свойства алкинов Autoionization of water Hydrolysis of salts
Autoionization of water Hydrolysis of salts ГИА. Вопрос 10. Химические свойства оксидов: основных, амфотерных, кислотных
ГИА. Вопрос 10. Химические свойства оксидов: основных, амфотерных, кислотных Предмет химии. Вещества. Изучаемые понятия: Вещество и тело. Атом и молекула. Свойства вещ
Предмет химии. Вещества. Изучаемые понятия: Вещество и тело. Атом и молекула. Свойства вещ Энергетика химических процессов. Основы термохимии
Энергетика химических процессов. Основы термохимии Регуляция и интеграция обмена веществ
Регуляция и интеграция обмена веществ Действующие вещества. Лексикон
Действующие вещества. Лексикон Противогололёдные реагенты. Вред или польза?
Противогололёдные реагенты. Вред или польза? Евгений Шварц «Сказка о потерянном времени»: «… ты помни: человек, который понапрасну теряет время, сам не замечает, как стареет»
Евгений Шварц «Сказка о потерянном времени»: «… ты помни: человек, который понапрасну теряет время, сам не замечает, как стареет» Исследование свойств и применение в медицине алкалоидов. Извлечение алкалоидов из травы белены черной.
Исследование свойств и применение в медицине алкалоидов. Извлечение алкалоидов из травы белены черной. Изотопы. Ядерные реакции. (Тема 8)
Изотопы. Ядерные реакции. (Тема 8) ХТА пестицидов ФОС
ХТА пестицидов ФОС Окислительно-восстановительные реакции
Окислительно-восстановительные реакции Презентация по Химии "Химические волокна." - скачать смотреть бесплатно_
Презентация по Химии "Химические волокна." - скачать смотреть бесплатно_ Презентация по Химии "Полімери" - скачать смотреть бесплатно
Презентация по Химии "Полімери" - скачать смотреть бесплатно АЛЮМІНІЄВІ СПЛАВИ Підготувала учениця 10 класу Кисленко Єлизавета
АЛЮМІНІЄВІ СПЛАВИ Підготувала учениця 10 класу Кисленко Єлизавета  Аналитическая химия
Аналитическая химия Стимулсезімтал сополимерлердің полимерлік комплексін зерттеу
Стимулсезімтал сополимерлердің полимерлік комплексін зерттеу Карбоновые кислоты и их функциональные производные
Карбоновые кислоты и их функциональные производные Углеводы. Моносахариды
Углеводы. Моносахариды Современные методы образования амидной связи с использованием ацилгалогенидов, ангидридов, активированных эфиров и их аналогов
Современные методы образования амидной связи с использованием ацилгалогенидов, ангидридов, активированных эфиров и их аналогов Спирты
Спирты Викторина. Химический Элементариум
Викторина. Химический Элементариум Элективный курс "Здоровье, красота и химия"
Элективный курс "Здоровье, красота и химия"