Содержание
- 2. The ideal gas equation
- 3. Room temperature and pressure, RTP Limitations At RTP, 1 mol of gas molecules occupies 24.0 dm3
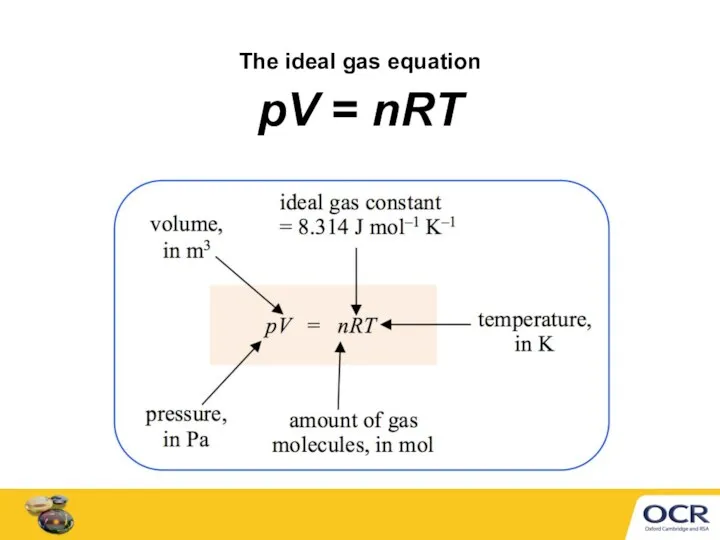
- 4. The ideal gas equation pV = nRT
- 5. Converting units for pV = nRT Before using pV = nRT, convert units to m3, K
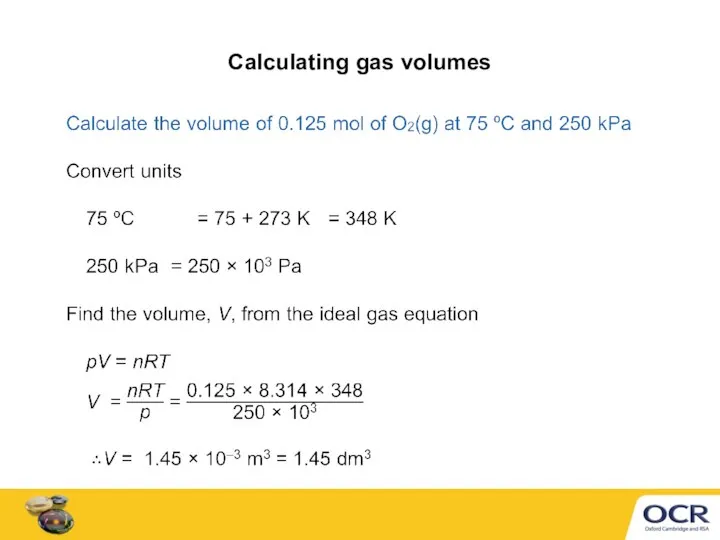
- 6. Calculating gas volumes
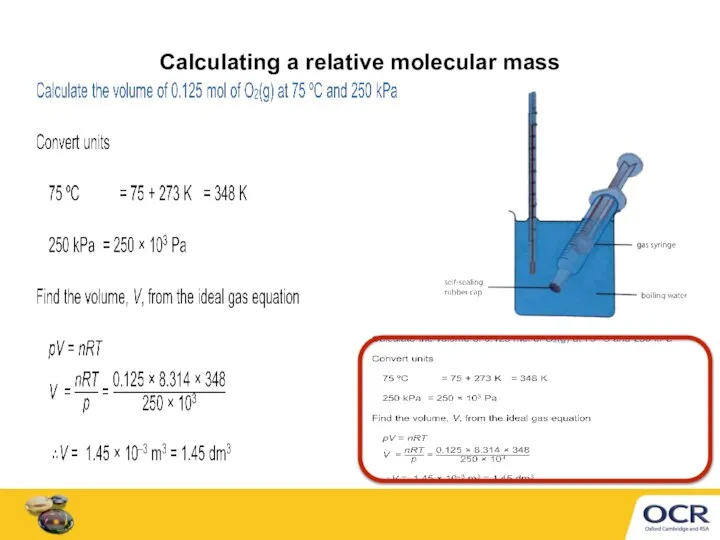
- 7. Calculating a relative molecular mass
- 9. AN INTRODUCTION TO ATOM ECONOMY KNOCKHARDY PUBLISHING
- 10. ATOM ECONOMY In most reactions you only want to make one of the resulting products Atom
- 11. ATOM ECONOMY In most reactions you only want to make one of the resulting products Atom
- 12. WORKED CALCULATIONS Calculate the atom economy for the formation of 1,2-dichloroethane, C2H4Cl2 Example 1
- 13. WORKED CALCULATIONS Calculate the atom economy for the formation of 1,2-dichloroethane, C2H4Cl2 Equation C2H4 + Cl2
- 14. WORKED CALCULATIONS Calculate the atom economy for the formation of nitrobenzene, C6H5NO2 Example 2
- 15. WORKED CALCULATIONS Calculate the atom economy for the formation of nitrobenzene, C6H5NO2 Equation C6H6 + HNO3
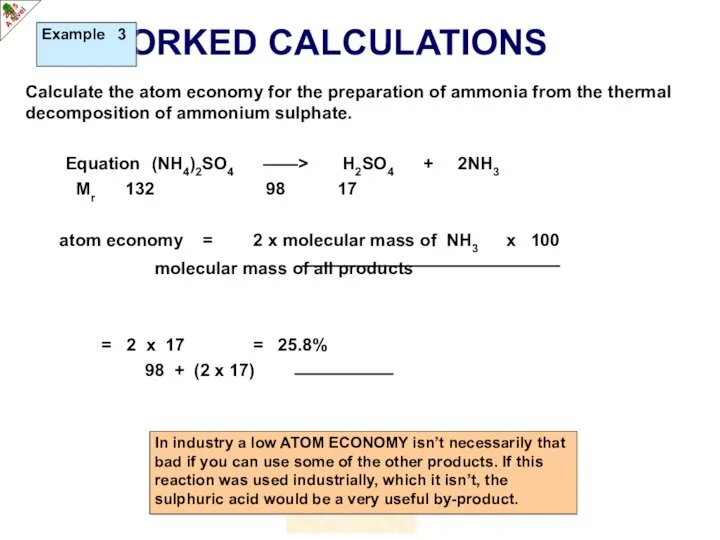
- 16. WORKED CALCULATIONS Calculate the atom economy for the preparation of ammonia from the thermal decomposition of
- 17. WORKED CALCULATIONS Calculate the atom economy for the preparation of ammonia from the thermal decomposition of
- 18. CALCULATIONS Calculate the atom economy of the following reactions (the required product is shown in red)
- 19. CALCULATIONS Calculate the atom economy of the following reactions (the required product is shown in red)
- 20. CALCULATIONS Calculate the atom economy of the following reactions (the required product is shown in red)
- 21. OVERVIEW • addition reactions will have 100% atom economy • substitution reactions will have less than
- 22. Perform calculations to determine the percentage yield of a reaction Percentage yield
- 23. In a chemical reaction which is totally efficient all the REACTANTS are converted into products. This
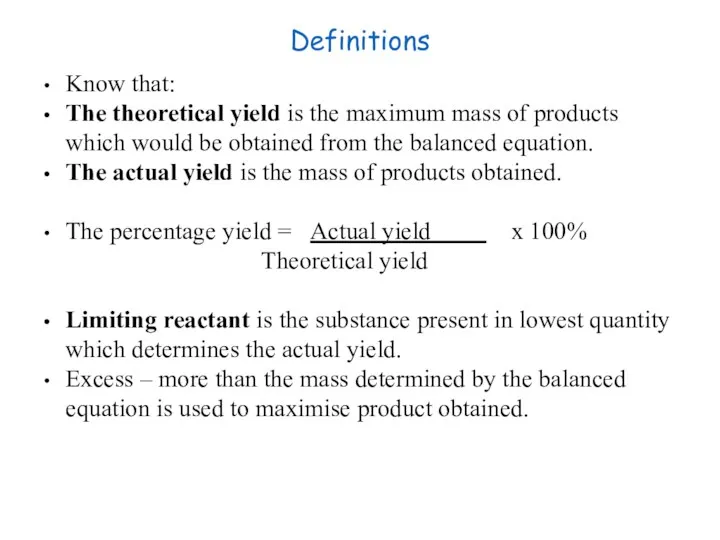
- 24. Definitions Know that: The theoretical yield is the maximum mass of products which would be obtained
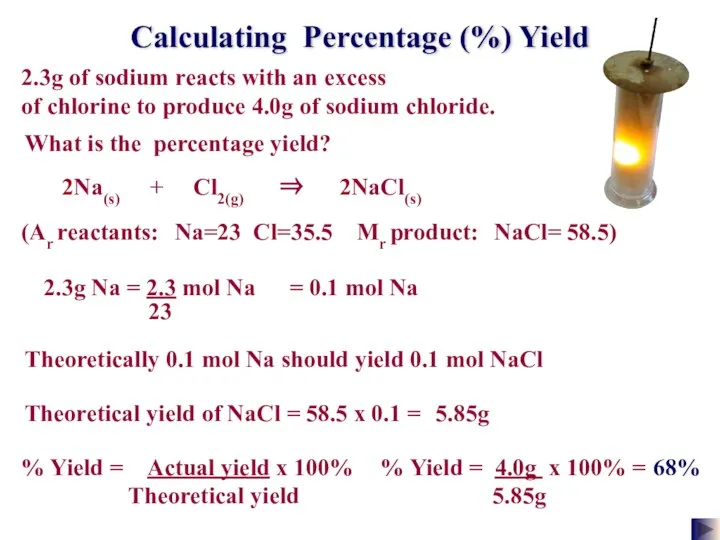
- 25. Calculating Percentage (%) Yield 2.3g of sodium reacts with an excess of chlorine to produce 4.0g
- 26. Calculating Percentage (%) Yield If 1.2g of magnesium reacts with an excess of oxygen to produce
- 28. Скачать презентацию

















 Простые и сложные вещества. Металлы и неметаллы. Бинарные соединения
Простые и сложные вещества. Металлы и неметаллы. Бинарные соединения Понятие фазы вещества. Насыщенный пар и его свойства. Влажность воздуха. Приборы для измерения влажности воздуха
Понятие фазы вещества. Насыщенный пар и его свойства. Влажность воздуха. Приборы для измерения влажности воздуха Полимерные материалы
Полимерные материалы Презентация по Химии "Сміття 1" - скачать смотреть
Презентация по Химии "Сміття 1" - скачать смотреть  Химический состав свежих плодов и овощей
Химический состав свежих плодов и овощей Готовимся к контрольной работе по химии
Готовимся к контрольной работе по химии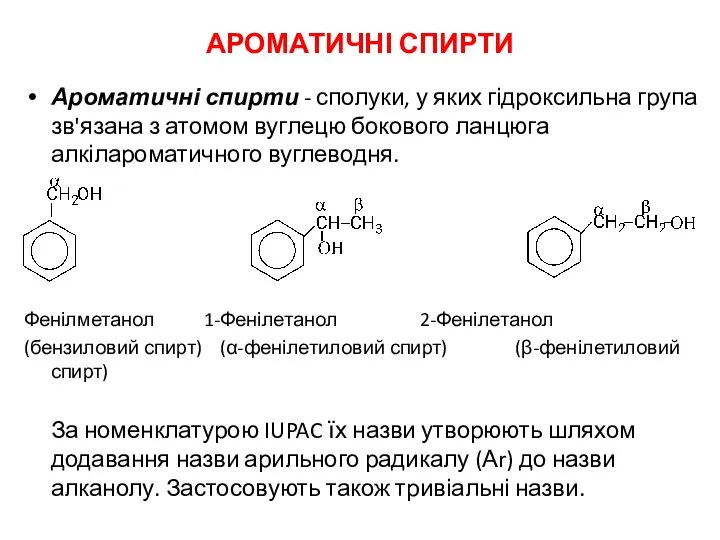 Ароматичні спирти
Ароматичні спирти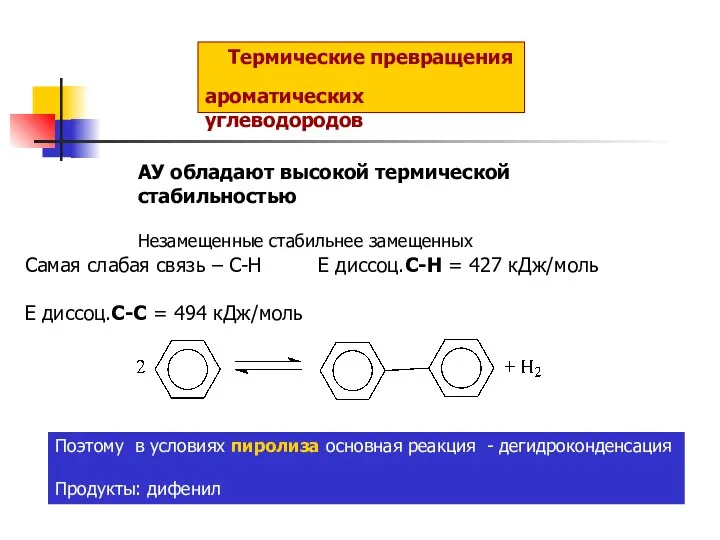 Термические превращения ароматических углеводородов
Термические превращения ароматических углеводородов Минералы натрия
Минералы натрия Алканы
Алканы Поверхностные явления. Лекция 15
Поверхностные явления. Лекция 15 Камни и Лев
Камни и Лев Гетерофункциональные органические соединения. Определение
Гетерофункциональные органические соединения. Определение Химическая посуда и ее значение (1)
Химическая посуда и ее значение (1) Материаловедение. Химические волокна (7 класс)
Материаловедение. Химические волокна (7 класс) Свойства растворов
Свойства растворов Аттестационная работа. Проектная и исследовательская деятельность как способ формирования химических знаний и умений
Аттестационная работа. Проектная и исследовательская деятельность как способ формирования химических знаний и умений Натуральный яблочный уксус
Натуральный яблочный уксус Губна помада та ії призначення
Губна помада та ії призначення Углерод. 9 класс
Углерод. 9 класс Электронная формула химических элементов
Электронная формула химических элементов Кислород. Характеристика и применение
Кислород. Характеристика и применение Строение соединений d-элементов
Строение соединений d-элементов Қалдық мөлшерлер. Бромды метил буының концентрациясын анықтау тәсілдемесі
Қалдық мөлшерлер. Бромды метил буының концентрациясын анықтау тәсілдемесі Обменные реакции в растворах электролитов
Обменные реакции в растворах электролитов Сложное вещество
Сложное вещество Cложные липиды (липоиды)
Cложные липиды (липоиды) Азот. Химические свойства азота
Азот. Химические свойства азота