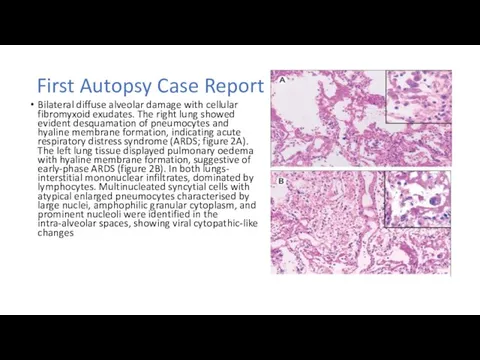
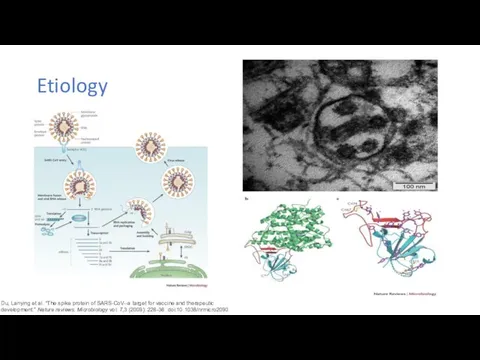
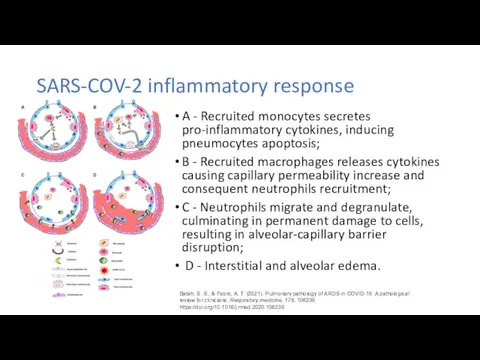
First Autopsy Case Report
Xu Z, Shi L, Wang Y, Zhang J,
Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 Apr;8(4):420-422.
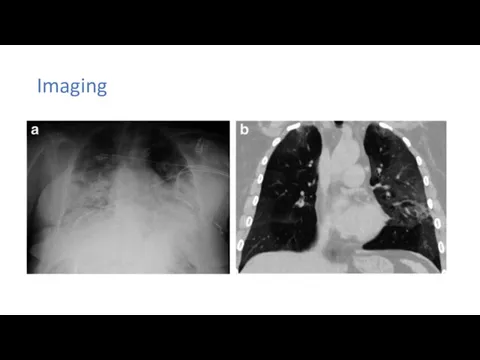
A 50-year-old man was admitted to a fever clinic on Jan 21, 2020, with symptoms of fever, chills, cough, fatigue and shortness of breath. He reported a travel history to Wuhan Jan 8–12, and that he had initial symptoms of mild chills and dry cough on Jan 14 (day 1 of illness) but did not see a doctor and kept working until Jan 21. Chest x-ray showed multiple patchy shadows in both lungs, and a throat swab sample was taken. On Jan 22 (day 9 of illness), the Beijing Centers for Disease Control (CDC) confirmed by reverse real-time PCR assay that the patient had COVID-19.



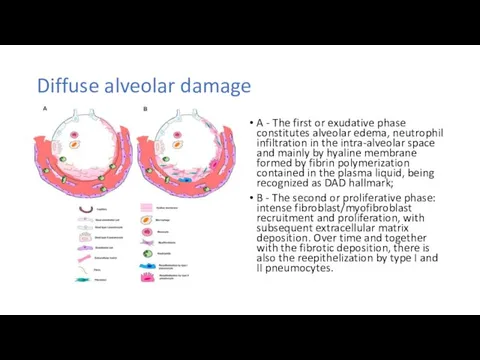
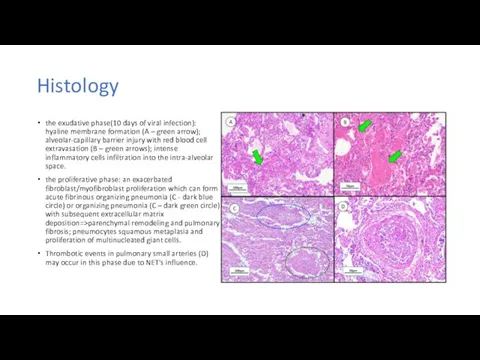
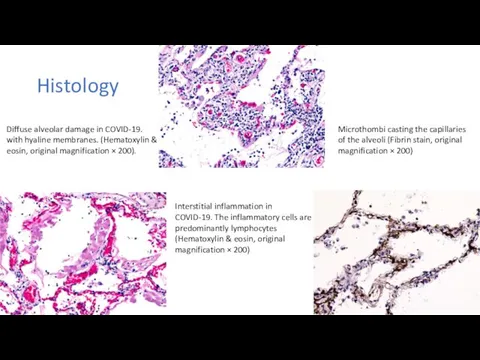
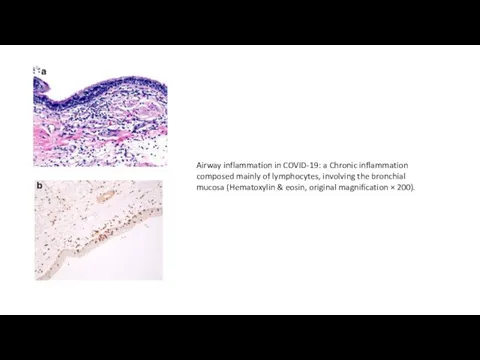


 Методика проведения искусственного дыхания
Методика проведения искусственного дыхания Введение в патофизиологию
Введение в патофизиологию Қозу
Қозу Острая почечная недостаточность
Острая почечная недостаточность Репродуктивное здоровье
Репродуктивное здоровье Доброкачественные и злокачественные опухоли почек
Доброкачественные и злокачественные опухоли почек Фарингоспрей заботиться о горле
Фарингоспрей заботиться о горле Клиническая генетика
Клиническая генетика Дифференциальная диагностика симптомов портальной гипертензии
Дифференциальная диагностика симптомов портальной гипертензии Дерматологические аспекты ВИЧ-инфекции
Дерматологические аспекты ВИЧ-инфекции Антимикробные лекарстенные средства
Антимикробные лекарстенные средства Внематочная беременность – новый взгляд
Внематочная беременность – новый взгляд Созылмалы аурулармен балаларды диспансерлік бақылаудың принциптері
Созылмалы аурулармен балаларды диспансерлік бақылаудың принциптері Холера. Этиология. Эпидемиология. Механизм заражения холерой
Холера. Этиология. Эпидемиология. Механизм заражения холерой БЛХ. Гипоталамо-гипофизарные заболевания
БЛХ. Гипоталамо-гипофизарные заболевания Виды и типы ортодонтических аппаратов и основы ортодонтического лечения
Виды и типы ортодонтических аппаратов и основы ортодонтического лечения Вирус иммунодефицита человека
Вирус иммунодефицита человека Особенности психики детей дошкольного возраста
Особенности психики детей дошкольного возраста Артериальная гипертензия
Артериальная гипертензия Производные аминоалкилбензолов. (Тема 3)
Производные аминоалкилбензолов. (Тема 3) Основы эндоурологии. Основные инструменты в эндоурологии
Основы эндоурологии. Основные инструменты в эндоурологии Новый способ восстановления непрерывности пищеварительного тракта после гастрэктомии
Новый способ восстановления непрерывности пищеварительного тракта после гастрэктомии Сүйек буын аурулары зақымданғанда визуалды диагностика
Сүйек буын аурулары зақымданғанда визуалды диагностика Применение различных средств индивидуальной защиты у больных и контактных covid-19
Применение различных средств индивидуальной защиты у больных и контактных covid-19 Контроль диабета при беременности
Контроль диабета при беременности Методы мозгового штурма
Методы мозгового штурма Нейропсихологические синдромы поражения теменной области головного мозга
Нейропсихологические синдромы поражения теменной области головного мозга Оформление медицинской документации
Оформление медицинской документации