Содержание
- 2. By the end of this presentation viewers should be able to describe the following about the
- 3. Vaccine Adverse Event Definition Adverse events are defined as health effects that occur after immunization that
- 4. Vaccine Adverse Event Reporting System (VAERS) National spontaneous reporting system for adverse events after US-licensed vaccines
- 5. Purpose of VAERS VAERS is used to: Identify new and/or rare adverse events following immunization Monitor
- 6. VAERS Strengths Can detect very rare adverse events that may not be detected before licensure Generates
- 7. VAERS Limitations Underreporting Stimulated reporting due to media attention and other factors Possibly incomplete and inaccurate
- 8. VAERS Uses (Examples) General Safety of Vaccines H1N1 influenza vaccines Safety of Influenza A (H1N1) 2009
- 9. VAERS Uses (Examples) continued Reassuring Evidence Supporting Vaccine Safety Guillain-Barre Syndrome risk identified following 1976 influenza
- 10. What to Report to VAERS Report any clinically significant adverse event following immunization (www.vaers.hhs.gov) Even if
- 11. What to Report to VAERS (continued) The report asks for information about pt, provider and reporter
- 12. How to Submit a VAERS Report: One of Several Methods May Be Used Online via a
- 13. VAERS Follow-up VAERS staff follow up with health care providers on serious reports and certain selected
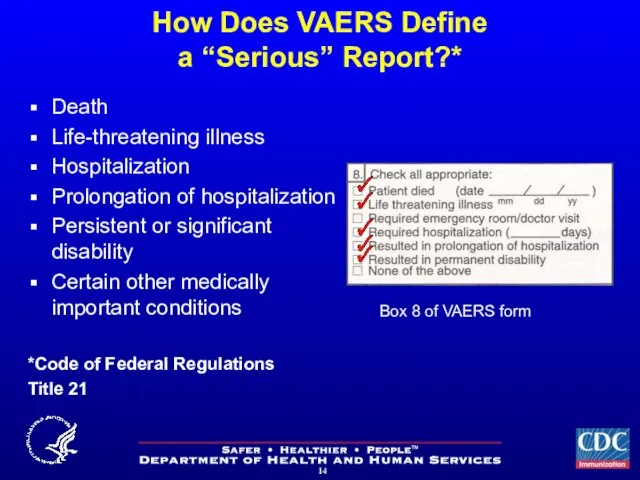
- 14. How Does VAERS Define a “Serious” Report?* Death Life-threatening illness Hospitalization Prolongation of hospitalization Persistent or
- 15. Selected Questions and Answers about VAERS
- 16. How are VAERS Reports Analyzed? CDC and FDA have primary responsibility for analysis Assess for signals
- 17. How Can Public VAERS Data Be Obtained? VAERS data (without identifiable personal information) are accessible to
- 18. What Are the Best Resources for Vaccine Safety? Publications updated with vaccine safety research findings and
- 19. How Can Healthcare and Vaccine Providers Contribute to Vaccine Safety? Properly store and administer vaccine http://www.cdc.gov/vaccines/recs/storage/default.htm
- 20. Continued: How Can Healthcare and Vaccine Providers Contribute to Vaccine Safety? Patient Education Materials CDC Vaccine
- 21. HHS Vaccine Safety Resources CDC Immunization Safety Office Web site www.cdc.gov/vaccinesafety 800-CDC-INFO (232-4636) CDCinfo@cdc.gov VAERS -CDC/FDA
- 22. VAERS Selected Bibliography Varricchio F, Iskander J, Destefano F, Ball R, Pless R, Braun MM, Chen
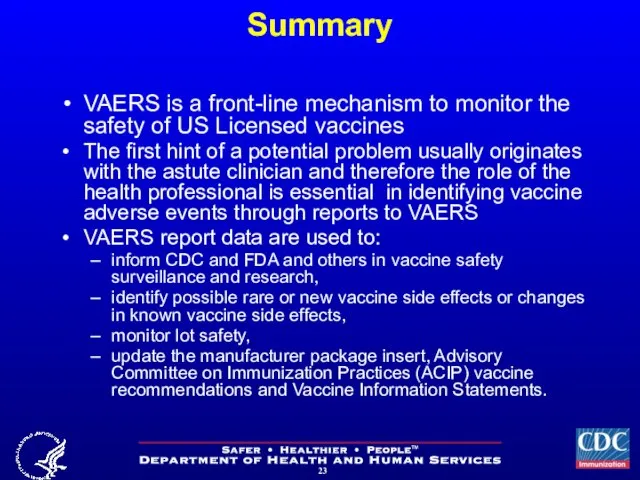
- 23. Summary VAERS is a front-line mechanism to monitor the safety of US Licensed vaccines The first
- 24. Questions
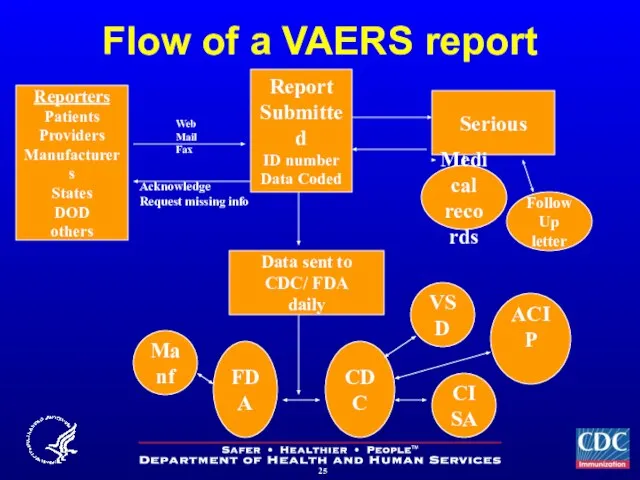
- 25. Flow of a VAERS report Report Submitted ID number Data Coded Serious Medical records Data sent
- 26. VAERS Background US post licensure vaccine safety surveillance Collects voluntary reports of adverse events following immunization
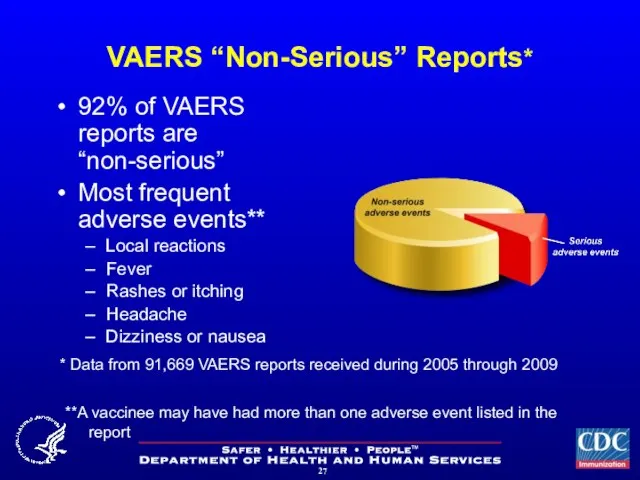
- 27. VAERS “Non-Serious” Reports* 92% of VAERS reports are “non-serious” Most frequent adverse events** Local reactions Fever
- 29. Скачать презентацию






















 Ишемическая болезнь сердца (инфаркт миокарда)
Ишемическая болезнь сердца (инфаркт миокарда) Гипоксия түсінігімен таныстырып, оның түрлерін, пайда болу себептері мен даму жолдарын және организмде
Гипоксия түсінігімен таныстырып, оның түрлерін, пайда болу себептері мен даму жолдарын және организмде Патогенные спирохеты – возбудители сифилиса, боррелиозов и лептоспироза
Патогенные спирохеты – возбудители сифилиса, боррелиозов и лептоспироза Офтальмологический центр
Офтальмологический центр Каротидный синус
Каротидный синус Этил спирті. Алкогализмнің әлеуметтік мәселелері
Этил спирті. Алкогализмнің әлеуметтік мәселелері Предмет, задачи, методы психологии. Психология в практической деятельности врача. Психика и сознание. Формы проявления психики
Предмет, задачи, методы психологии. Психология в практической деятельности врача. Психика и сознание. Формы проявления психики Организация сестринского ухода за пациентами с гипертонической болезнью
Организация сестринского ухода за пациентами с гипертонической болезнью Содержимое аптечки, первичный комплекс сердечно-лёгочной реанимации (СЛР), первая помощь при неотложных состояниях
Содержимое аптечки, первичный комплекс сердечно-лёгочной реанимации (СЛР), первая помощь при неотложных состояниях Противовирусные препараты
Противовирусные препараты Заболевания сердечно-сосудистой системы
Заболевания сердечно-сосудистой системы Первые признаки инсульта
Первые признаки инсульта Нефростома кезіндегі пациент күтімі
Нефростома кезіндегі пациент күтімі Антигены крови
Антигены крови Молекулярные основы наследственности
Молекулярные основы наследственности Патогенные кокки
Патогенные кокки Первая помощь при синдроме длительного сдавливания, травматическом шоке. Тема 10
Первая помощь при синдроме длительного сдавливания, травматическом шоке. Тема 10 Лунатизм. Парасомнии
Лунатизм. Парасомнии Полиоксидоний. Борьба с простудой и гриппом
Полиоксидоний. Борьба с простудой и гриппом Новое расписание приёма в больницах г. Иркутска
Новое расписание приёма в больницах г. Иркутска Функциональная анатомия (остеология, миология)
Функциональная анатомия (остеология, миология) Сухожилия и как с ними бороться
Сухожилия и как с ними бороться Визуальная диагностика заболеваний пищеварительной системы
Визуальная диагностика заболеваний пищеварительной системы Скелет верхних и нижних конечночтей. Кости и их соединения
Скелет верхних и нижних конечночтей. Кости и их соединения Первая помощь ожогах
Первая помощь ожогах Система Егиз
Система Егиз Дене және сезім мүшелерінің ақауы бар науқастармен арақатынас құру
Дене және сезім мүшелерінің ақауы бар науқастармен арақатынас құру Подготовка к инструмент методам исследования
Подготовка к инструмент методам исследования