Содержание
- 2. What is Bioremediation?? Using subsurface microorganisms to transform hazardous contaminants into relatively harmless byproducts, such as
- 3. Bioremediation Background Natural Attenuation is Not fast enough, Not complete enough, Not frequently occurring enough to
- 4. Historical Perspective ~1900 Advent of biological processes to treat organics derived from human or animal wastes
- 5. Soil and Subsurface Contaminants Benzene and related fuel components (BTEX) Pyrene and other polynuclear aromatics Chlorinated
- 6. Sources of Contamination Industrial spills and leaks Surface impoundments Storage tanks and pipes Landfills Burial areas
- 7. Current Water Issues Associated with Gasoline Use Widespread contamination Major treat to drinking water resources Components
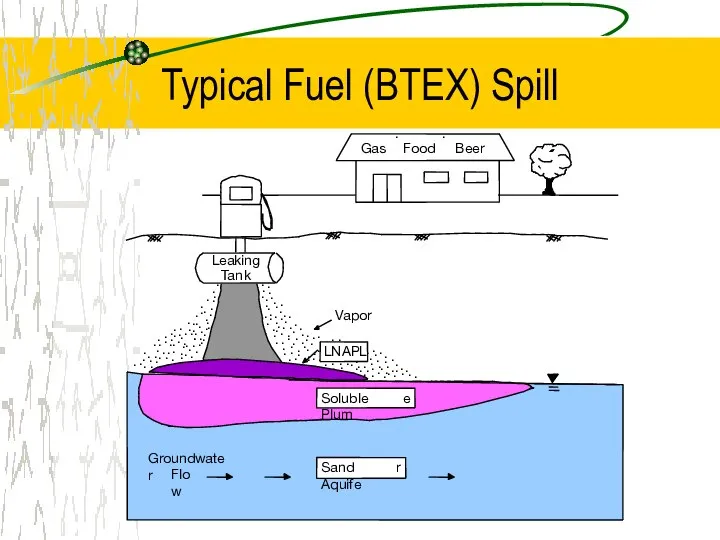
- 8. Typical Fuel (BTEX) Spill
- 9. Chlorinated Background Groundwater plumes of chlorinated solvents are widespread due to their extensive use at industrial,
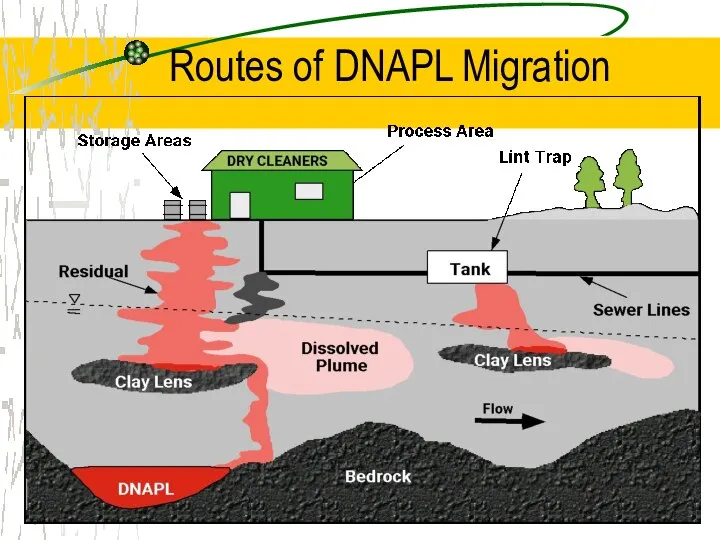
- 10. Routes of DNAPL Migration
- 11. DNAPL Our Most Difficult Challenge DNAPL source Residual phase Trapped on lenses Pools in low areas
- 12. Treatment Techniques Soil Extraction Pump and Treat Physical and/or reactive barriers Air and Hydrogen Sparging Biological
- 13. Why use Bioremediation? No additional disposal costs Low maintenance Does not create an eyesore Capable of
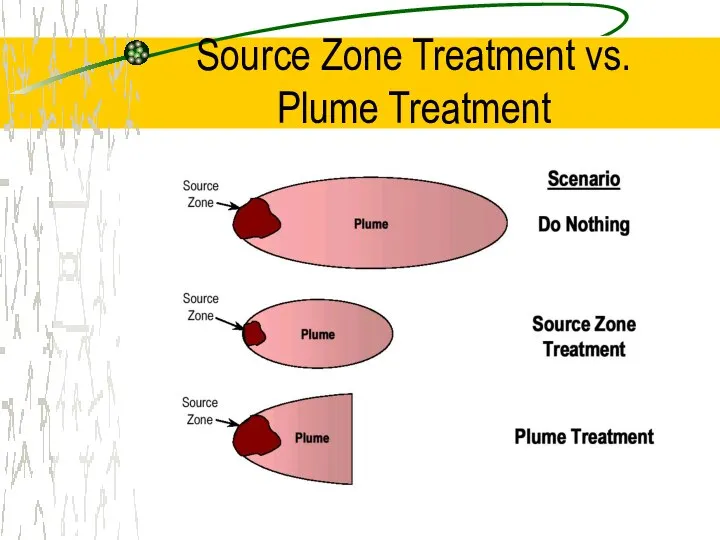
- 14. Source Zone Treatment vs. Plume Treatment
- 15. Fundamentals of Biodegradation All organics are biodegradable, BUT biodegradation requires specific conditions There is no Superbug
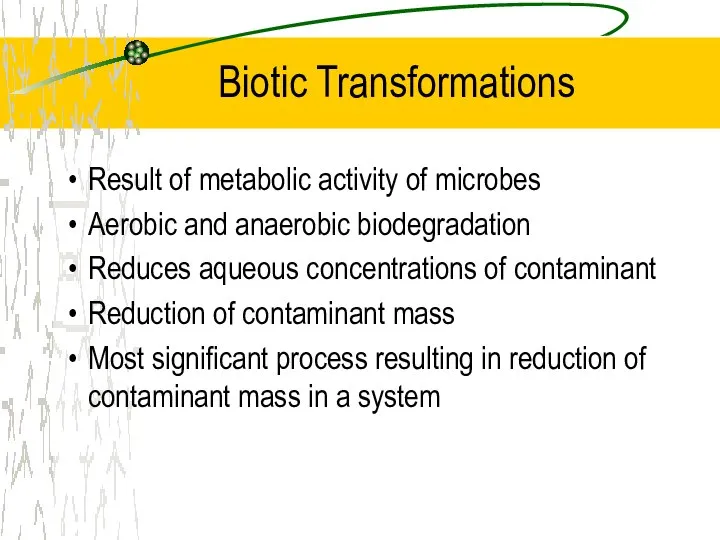
- 16. Biotic Transformations Result of metabolic activity of microbes Aerobic and anaerobic biodegradation Reduces aqueous concentrations of
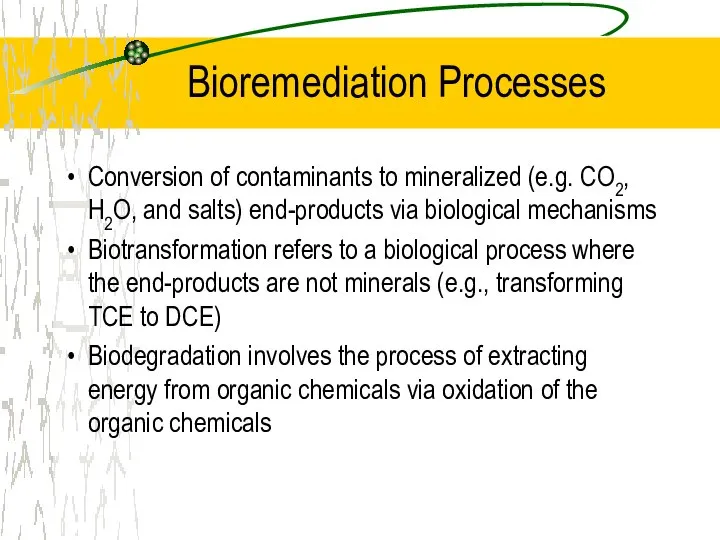
- 17. Bioremediation Processes Conversion of contaminants to mineralized (e.g. CO2, H2O, and salts) end-products via biological mechanisms
- 18. How Microbes Use the Contaminant Contaminants may serve as: Primary substrate enough available to be the
- 19. Requirements for Microbial Growth
- 20. Electron Exchange
- 21. Aerobic v. Anaerobic If oxygen is the terminal electron acceptor, the process is called aerobic biodegradation
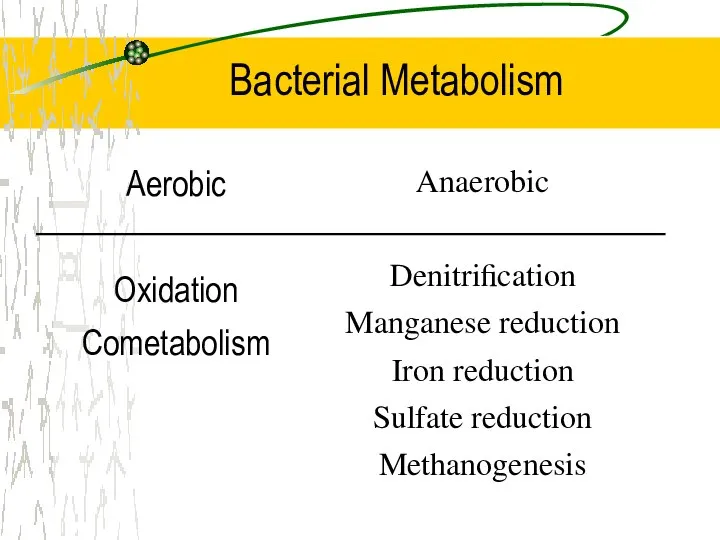
- 22. Aerobic Oxidation Cometabolism Anaerobic Denitrification Manganese reduction Iron reduction Sulfate reduction Methanogenesis Bacterial Metabolism
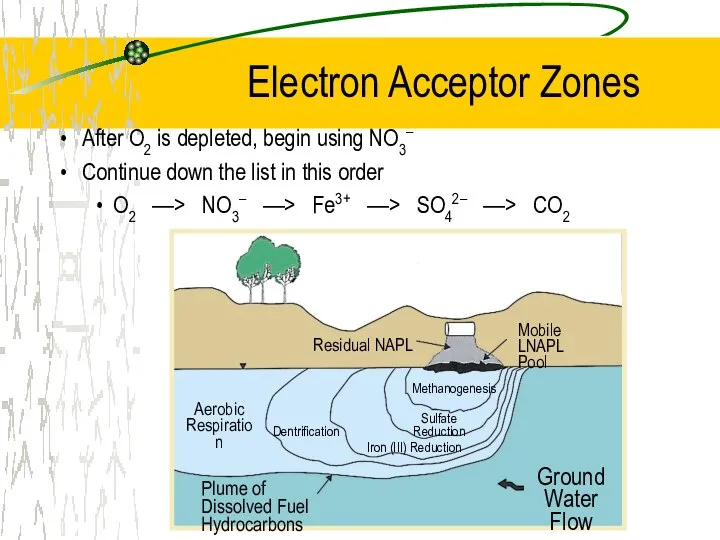
- 23. Electron Acceptor Zones After O2 is depleted, begin using NO3– Continue down the list in this
- 24. Electron Acceptor Condition
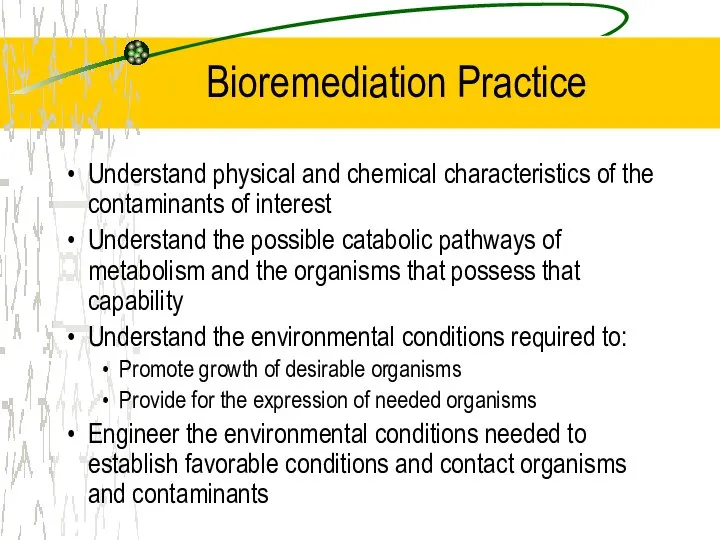
- 25. Bioremediation Practice Understand physical and chemical characteristics of the contaminants of interest Understand the possible catabolic
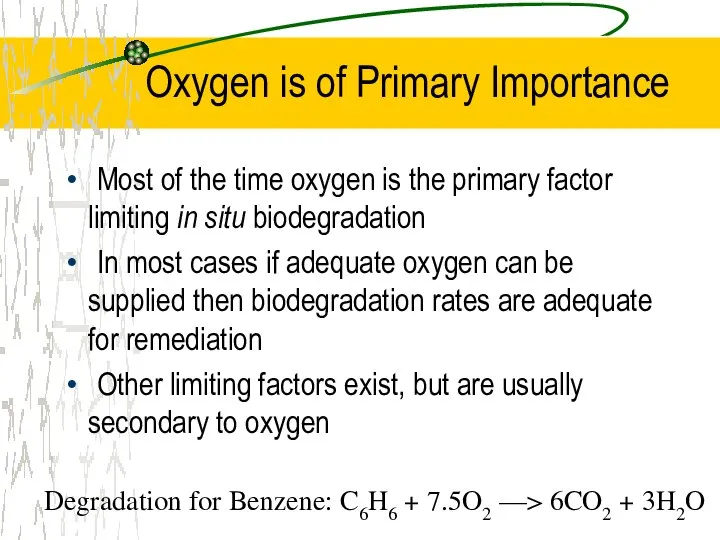
- 26. Oxygen is of Primary Importance Most of the time oxygen is the primary factor limiting in
- 27. Two ways to introduce oxygen in situ Dissolved in water : Actively pumped: H2 O2 ,
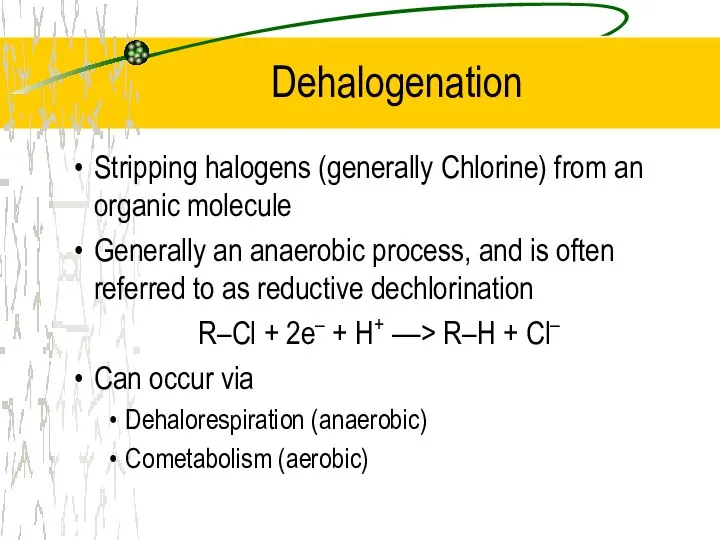
- 28. Dehalogenation Stripping halogens (generally Chlorine) from an organic molecule Generally an anaerobic process, and is often
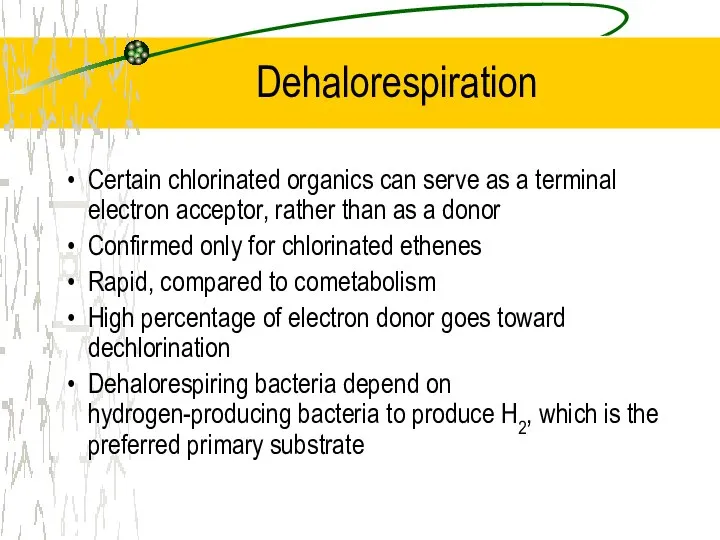
- 29. Dehalorespiration Certain chlorinated organics can serve as a terminal electron acceptor, rather than as a donor
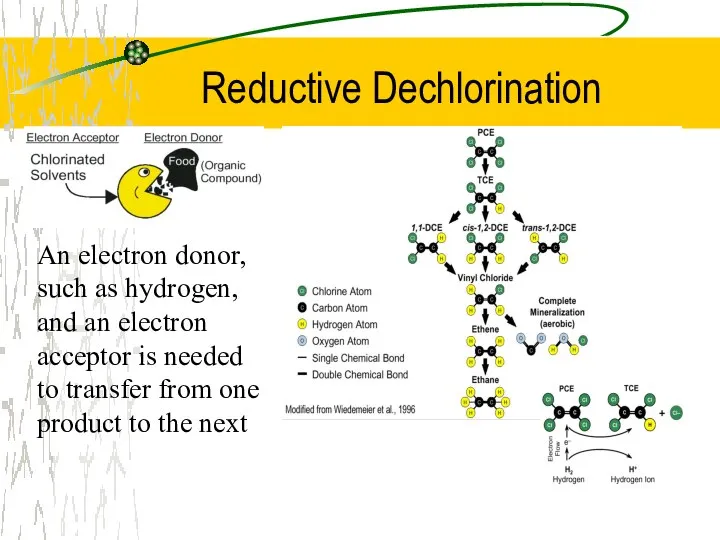
- 30. Reductive Dechlorination An electron donor, such as hydrogen, and an electron acceptor is needed to transfer
- 31. Added Danger Dechlorination of PCE and TCE should be encouraged, but monitored closely The dechlorination products
- 32. Cometabolism Fortuitous transformation of a compound by a microbe relying on some other primary substrate Generally
- 33. Selective Enhancement of Reductive Dechlorination Competition for available H2 in subsurface Dechlorinators can utilize H2 at
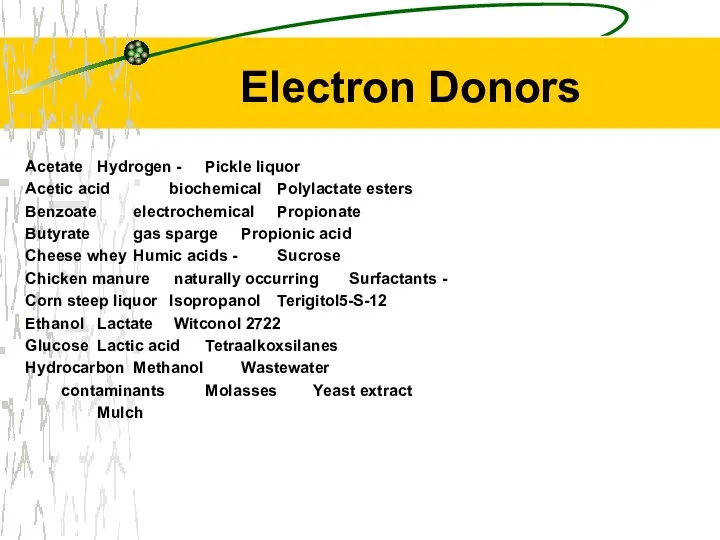
- 34. Electron Donors Alcohols and acids Almost any common fermentable compound Hydrogen apparently universal electron donor, but
- 35. Electron Donors Acetate Hydrogen - Pickle liquor Acetic acid biochemical Polylactate esters Benzoate electrochemical Propionate Butyrate
- 36. Enhanced Bioattenuation Petroleum Chlorinated Technology Hydrocarbons Solvents (e– acceptor) (e– donor) Liquid Delivery Oxygen Benzoate Nitrate
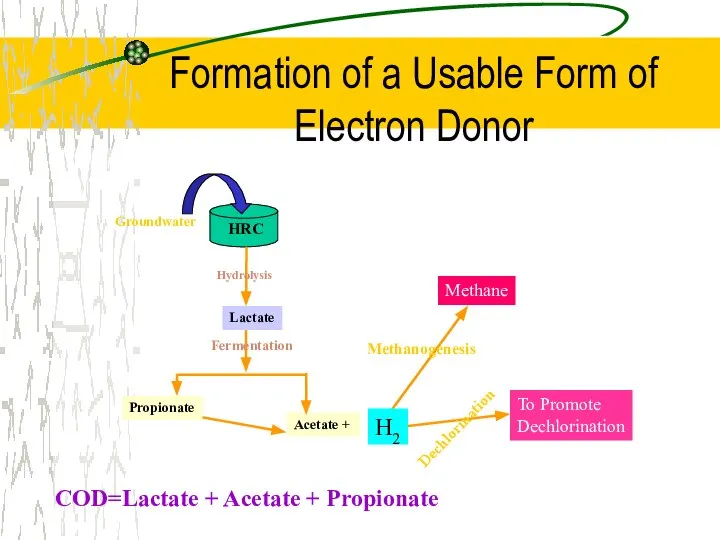
- 37. Formation of a Usable Form of Electron Donor COD=Lactate + Acetate + Propionate
- 39. Скачать презентацию














 Хищные птицы национального парка «Водлозерский», занесенные в Красную книгу Архангельской области
Хищные птицы национального парка «Водлозерский», занесенные в Красную книгу Архангельской области Исследуй снег. МКОУ "Ихальская СОШ", 1 класс. Команда "Природооткрыватели"
Исследуй снег. МКОУ "Ихальская СОШ", 1 класс. Команда "Природооткрыватели" Клуб Экомир (EcoMir Club)
Клуб Экомир (EcoMir Club) Вода - это жизнь
Вода - это жизнь Экология. Экологические факторы среды обитания
Экология. Экологические факторы среды обитания Сукцессии. Завершенность. Закономерности
Сукцессии. Завершенность. Закономерности Геоботаника - наука о растительном покрове, его строении, закономерностях распределения, связи с условиями местообитания
Геоботаника - наука о растительном покрове, его строении, закономерностях распределения, связи с условиями местообитания Учение о биосфере
Учение о биосфере Наша маленькая планета Земля. Защитим планету
Наша маленькая планета Земля. Защитим планету Экология. Концепция взаимоотношений общества и природы
Экология. Концепция взаимоотношений общества и природы Цепи и сети питания. Экологические пирамиды
Цепи и сети питания. Экологические пирамиды Роль живого вещества в природе
Роль живого вещества в природе Хельсинская конвенция
Хельсинская конвенция Экология. Загрязнение окружающей среды
Экология. Загрязнение окружающей среды Традиционные, альтернативные и необычные источники энергии
Традиционные, альтернативные и необычные источники энергии Экология жилища
Экология жилища Презентация Путешествие по Италии
Презентация Путешествие по Италии Дүниежүзі және Қазақстан Республикасының су қорының проблемалары
Дүниежүзі және Қазақстан Республикасының су қорының проблемалары Экономическая оценка вреда, причиняемого хозяйственной и иной деятельностью окружающей среде
Экономическая оценка вреда, причиняемого хозяйственной и иной деятельностью окружающей среде Компоненты биосферы
Компоненты биосферы Категорія «забруднення» навколишнього середовища. Формули та діаграми в Excel 2007
Категорія «забруднення» навколишнього середовища. Формули та діаграми в Excel 2007 Радиационные аварии и их последствия
Радиационные аварии и их последствия Кислотные осадки
Кислотные осадки Презентация На севере Европы
Презентация На севере Европы Презентация на тему Животные холодных и жарких стран
Презентация на тему Животные холодных и жарких стран  "Под куполом". Озоновый слой Земли
"Под куполом". Озоновый слой Земли Презентация Все о Загадках
Презентация Все о Загадках  Відходи виробництва та споживання товарів і проблеми їх утилізації
Відходи виробництва та споживання товарів і проблеми їх утилізації