Lecture 21
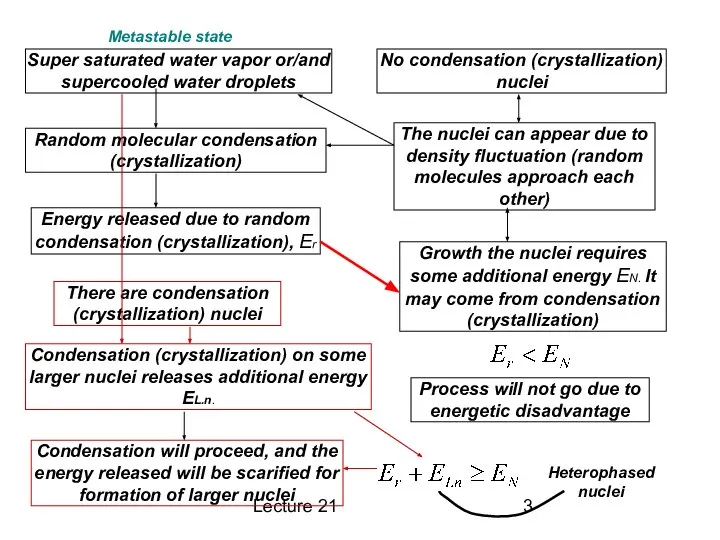
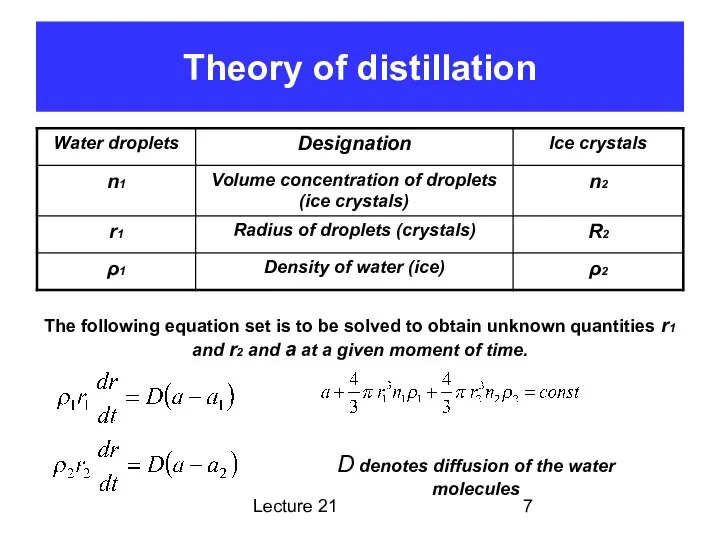
Super saturated water vapor or/and supercooled water droplets
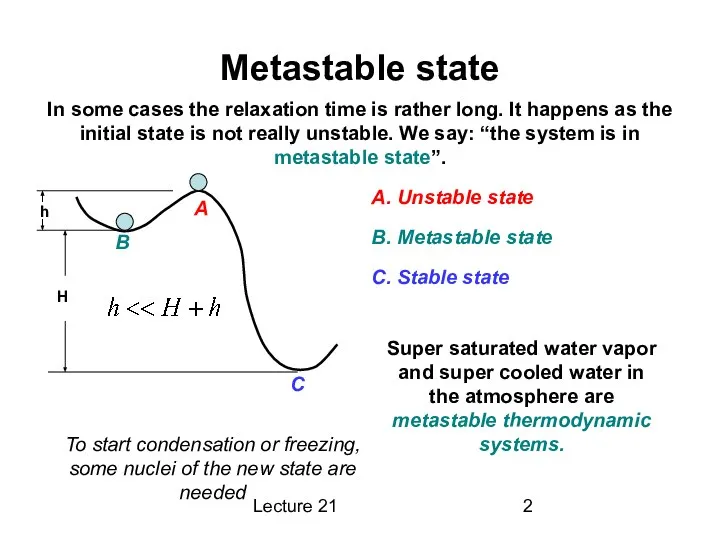
Metastable state
No condensation
(crystallization) nuclei
The nuclei can appear due to density fluctuation (random molecules approach each other)
Random molecular condensation (crystallization)
Energy released due to random condensation (crystallization), Er
Condensation (crystallization) on some larger nuclei releases additional energy EL.n.
Growth the nuclei requires some additional energy EN. It may come from condensation (crystallization)
Process will not go due to energetic disadvantage
There are condensation (crystallization) nuclei
Condensation will proceed, and the energy released will be scarified for formation of larger nuclei
Heterophased nuclei

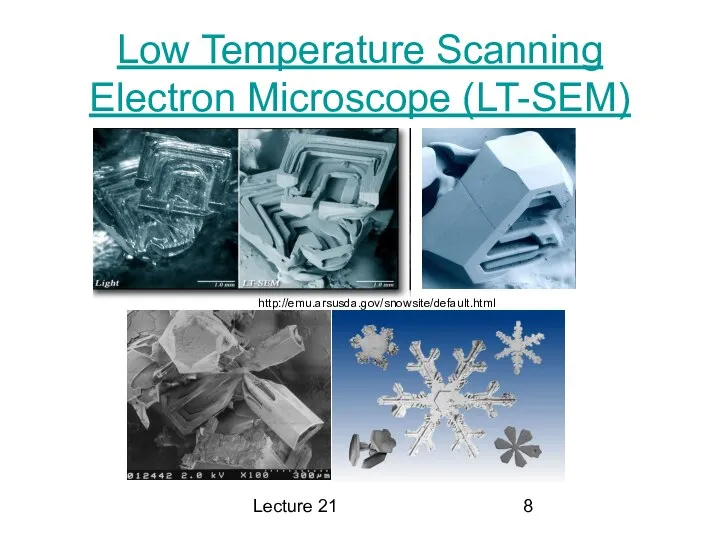


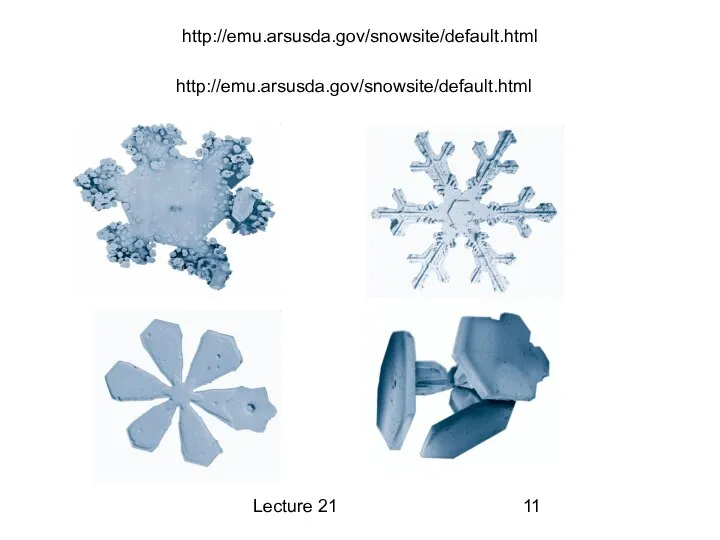
 Отчет о выполнении работ по благоустройству общественной территории. Вельский район деревня Никифорово
Отчет о выполнении работ по благоустройству общественной территории. Вельский район деревня Никифорово Политический режим
Политический режим Презентация Рынок недвижимости (РОССТАТ)
Презентация Рынок недвижимости (РОССТАТ)  Волгоградский Государственный Медицинский Университет Кафедра анатомии человека Общая артрология. Частная анатомия суст
Волгоградский Государственный Медицинский Университет Кафедра анатомии человека Общая артрология. Частная анатомия суст ХиТРРЭ.pptx
ХиТРРЭ.pptx Pneumatic devices
Pneumatic devices The Executive Branch
The Executive Branch  Группы риска макет
Группы риска макет Сахарный диабет 2 типа
Сахарный диабет 2 типа  Мой Петербург
Мой Петербург Массивы в Pascal. Одномерные массивы
Массивы в Pascal. Одномерные массивы Детско-юношеский отдых в регионах Татарстана. Лениногорский район
Детско-юношеский отдых в регионах Татарстана. Лениногорский район Инфекции, передаваемые половым путем
Инфекции, передаваемые половым путем Неоднозначность факторного решения
Неоднозначность факторного решения Курбан-байрам - праздник жертвоприношения
Курбан-байрам - праздник жертвоприношения Презентация на тему "Современный УМК как средство опережающего развития школьников в условиях обновления содержания образован
Презентация на тему "Современный УМК как средство опережающего развития школьников в условиях обновления содержания образован Информационно-исследовательский проект по музыке: «Влияние колокольного искусства на духовное возрождение общества в современны
Информационно-исследовательский проект по музыке: «Влияние колокольного искусства на духовное возрождение общества в современны Модификатор зеркало и создание разрезов
Модификатор зеркало и создание разрезов Проект на тему «Система работы с родителями в образовательном учреждении»
Проект на тему «Система работы с родителями в образовательном учреждении» Критика и самокритика
Критика и самокритика Общественное мнение и средства массовой информации
Общественное мнение и средства массовой информации Movie Industry in America
Movie Industry in America Административное право РК
Административное право РК Докторските студии -трет циклус во високото образование на РМ Проф. Д-р Марика Башеска – Ѓорѓиеска УКЛО, Економски факултет-Приле
Докторските студии -трет циклус во високото образование на РМ Проф. Д-р Марика Башеска – Ѓорѓиеска УКЛО, Економски факултет-Приле Работа в группа сентябрьх
Работа в группа сентябрьх Переход права собственности на жилое помещение в порядке договора купли продажи
Переход права собственности на жилое помещение в порядке договора купли продажи  Как свести к нулю количество конфликтов между проектной командой и командами поддержки или I believe in love. - презентация
Как свести к нулю количество конфликтов между проектной командой и командами поддержки или I believe in love. - презентация Буклет менеджера. Всероссийский форум breakpoint
Буклет менеджера. Всероссийский форум breakpoint