Содержание
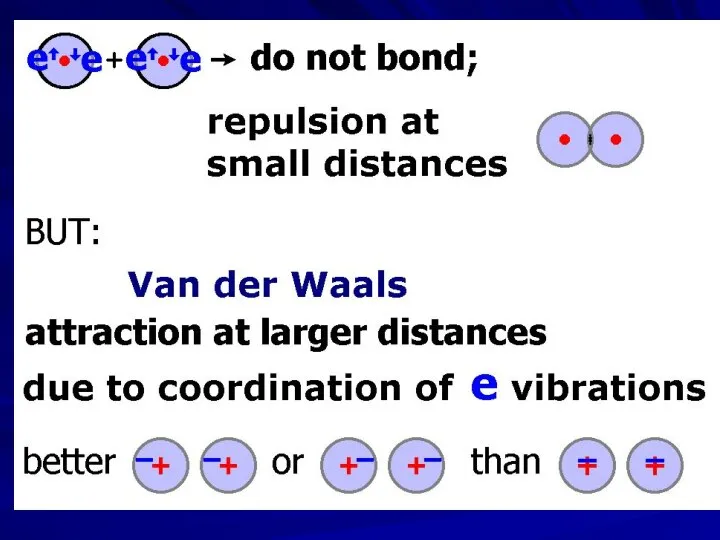
- 3. Johannes Diderik van der Waals (1837 – 1923) — Nobel Prize 1910 Fritz Wolfgang London (1900
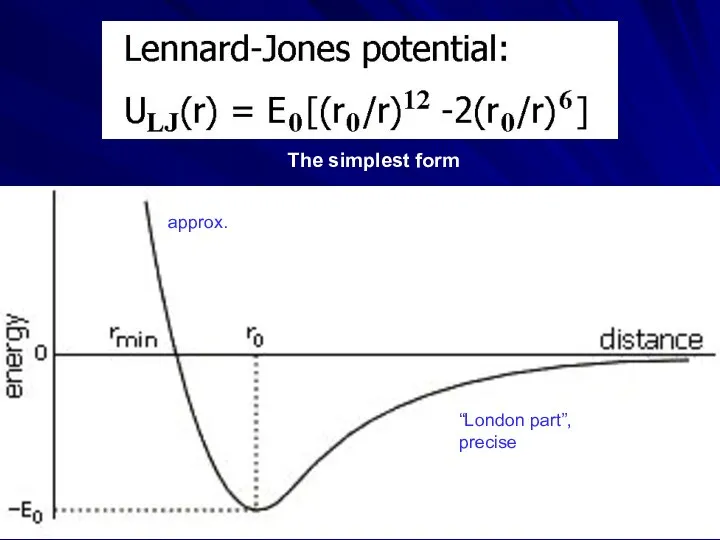
- 4. “London part”, precise approx. The simplest form
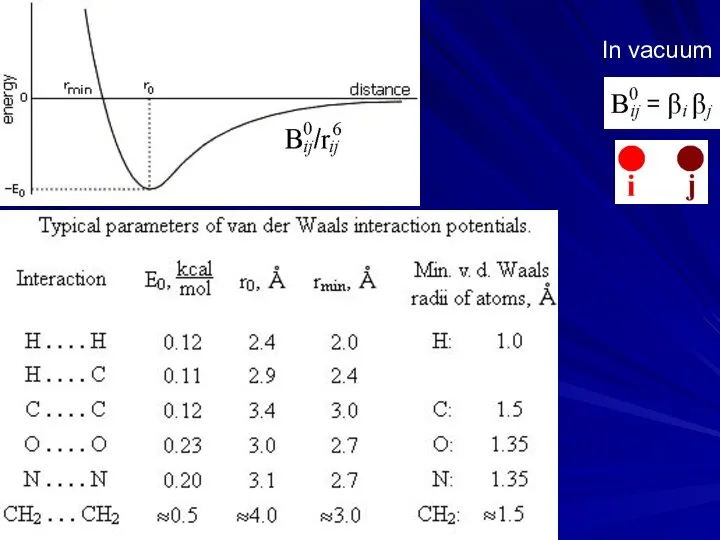
- 5. In vacuum
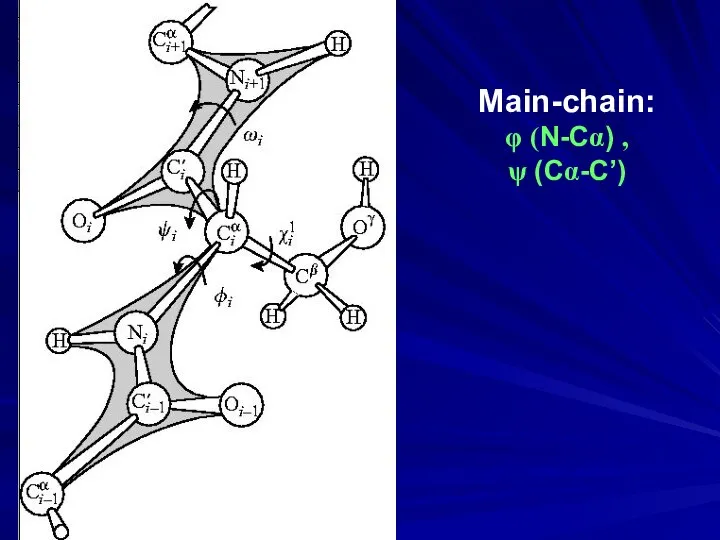
- 6. Main-chain: φ (N-Cα) , ψ (Cα-C’)
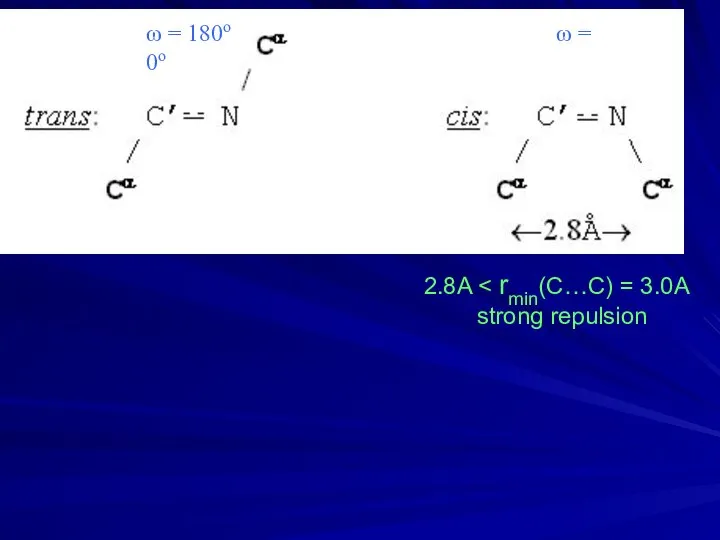
- 7. ω = 180ο ω = 0ο 2.8A strong repulsion
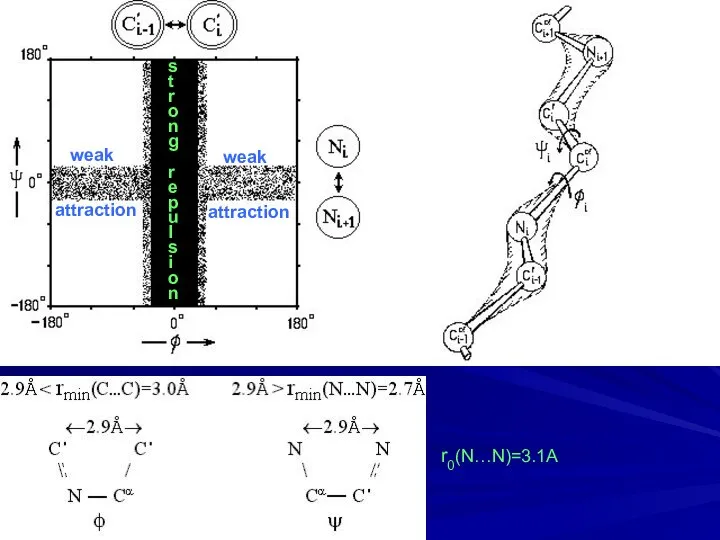
- 8. s t r o n g r e p u l s i o n weak
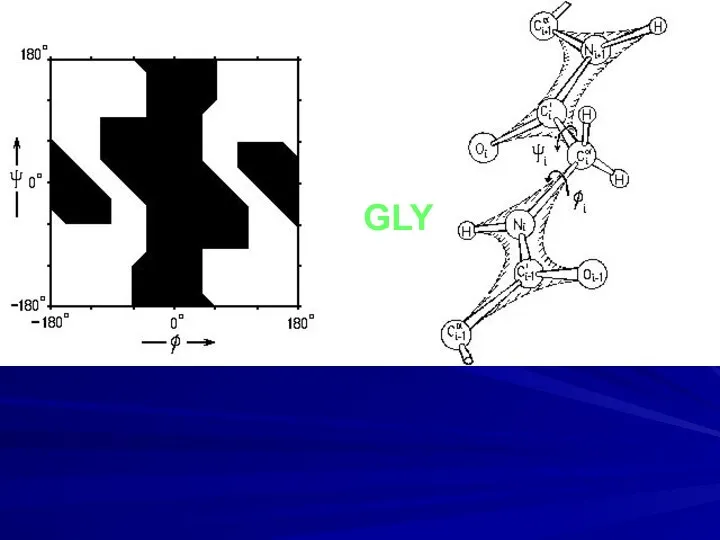
- 9. GLY
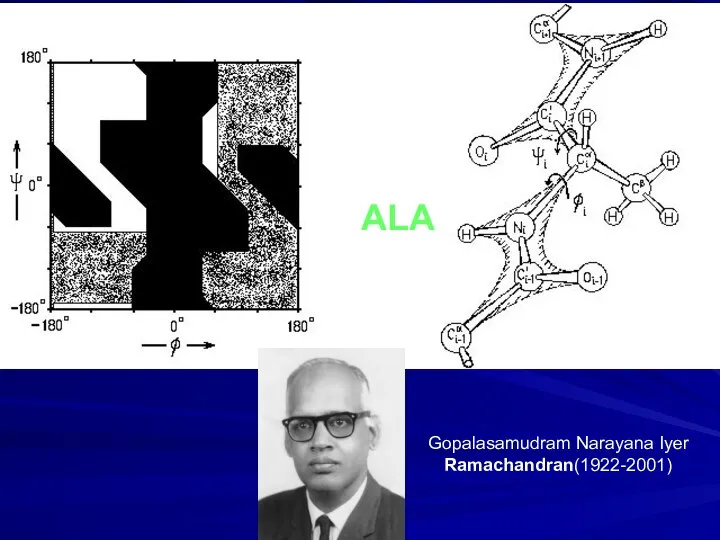
- 10. ALA Gopalasamudram Narayana Iyer Ramachandran(1922-2001)
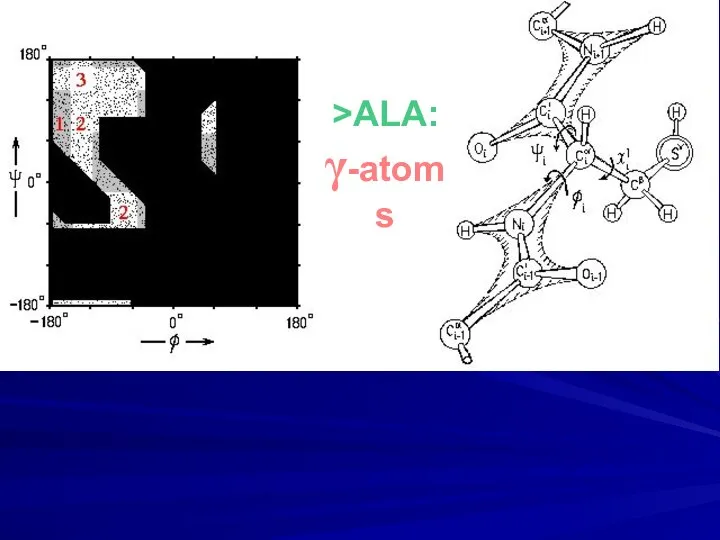
- 11. >ALA: γ-atoms
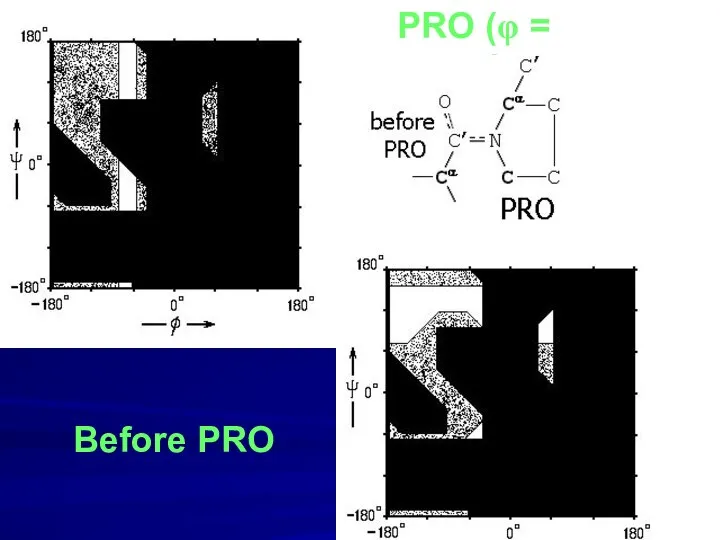
- 12. PRO (φ = -70o) Before PRO
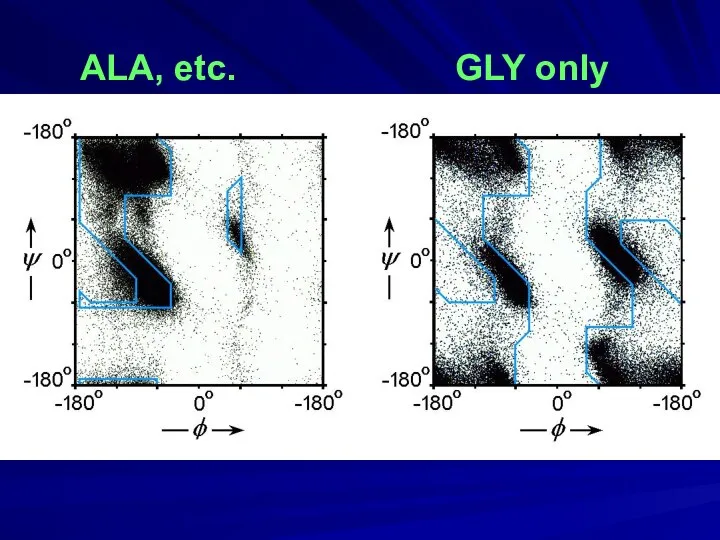
- 13. ALA, etc. GLY only
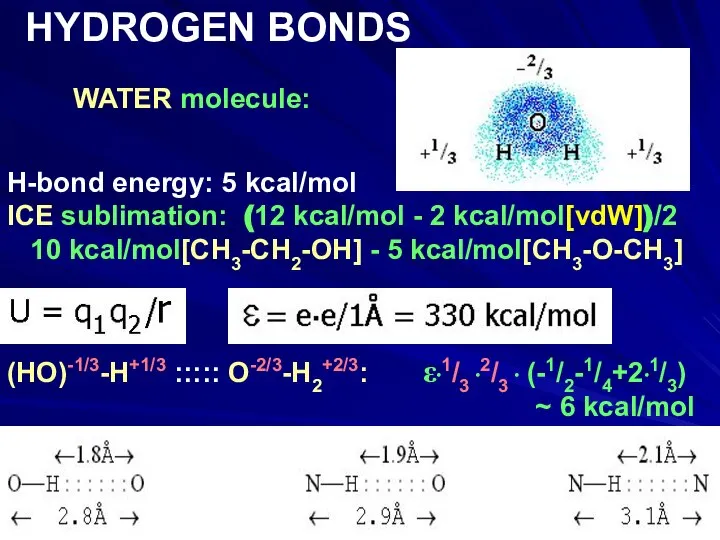
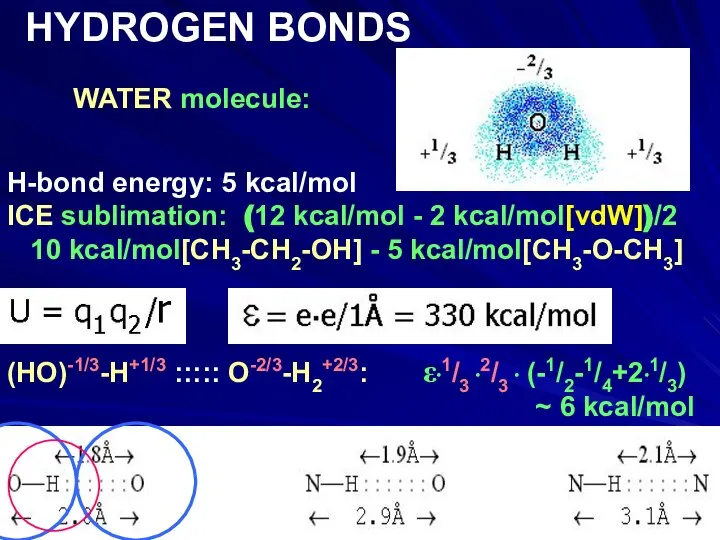
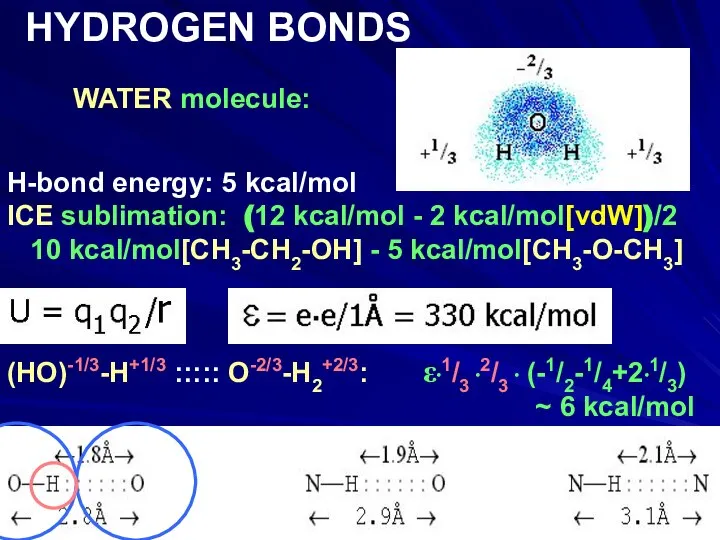
- 14. WATER molecule: HYDROGEN BONDS H-bond energy: 5 kcal/mol ICE sublimation: (12 kcal/mol - 2 kcal/mol[vdW])/2 10
- 15. WATER molecule: HYDROGEN BONDS H-bond energy: 5 kcal/mol ICE sublimation: (12 kcal/mol - 2 kcal/mol[vdW])/2 10
- 16. WATER molecule: HYDROGEN BONDS H-bond energy: 5 kcal/mol ICE sublimation: (12 kcal/mol - 2 kcal/mol[vdW])/2 10
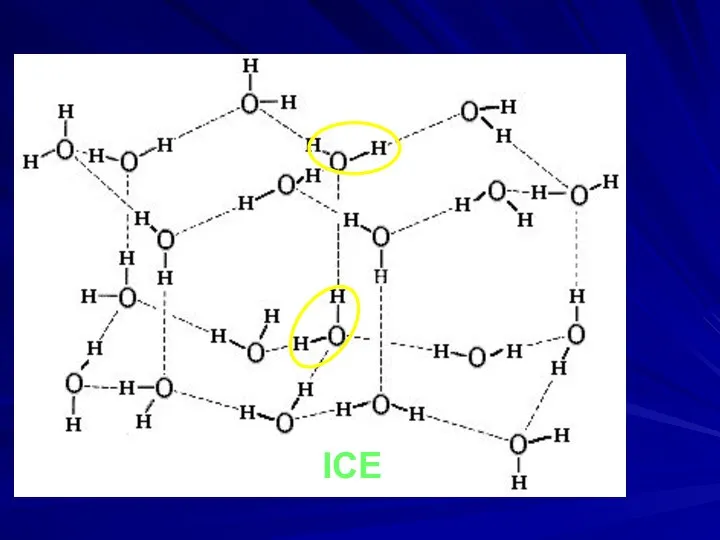
- 18. ICE
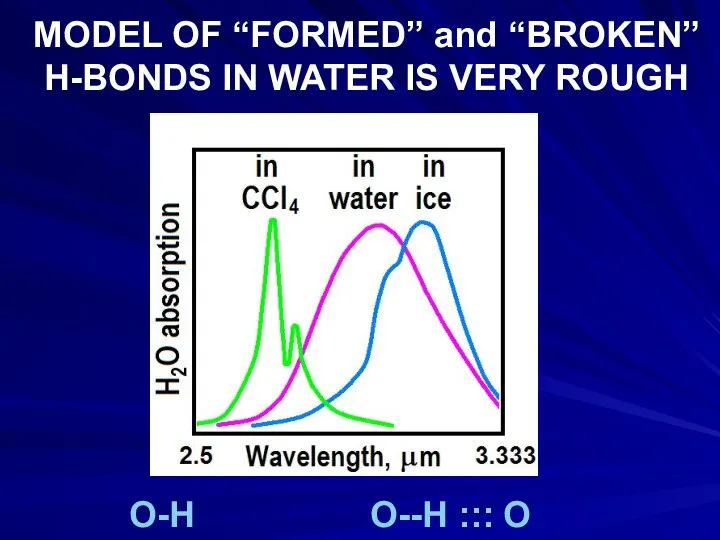
- 19. MODEL OF “FORMED” and “BROKEN” H-BONDS IN WATER IS VERY ROUGH O-H O--H ::: O
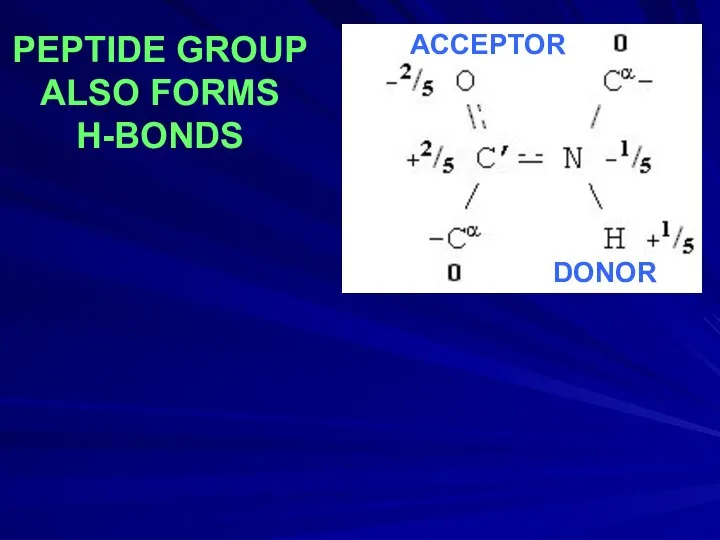
- 20. PEPTIDE GROUP ALSO FORMS H-BONDS DONOR ACCEPTOR
- 22. Скачать презентацию


 Презентация Масса тела. Единицы массы. Измерение массы на весах.
Презентация Масса тела. Единицы массы. Измерение массы на весах.  Сила трения
Сила трения Гибка - слесарная операция
Гибка - слесарная операция Нанотехнологии. Возникновение и развитие нанотехнологий
Нанотехнологии. Возникновение и развитие нанотехнологий Квантовая механика
Квантовая механика Elektriska piedzina. (№2)
Elektriska piedzina. (№2) Г. Фаренгейт, его вклад в развитие физики
Г. Фаренгейт, его вклад в развитие физики Презентация по физике "Электролиз растворов солей" - скачать бесплатно
Презентация по физике "Электролиз растворов солей" - скачать бесплатно Законы сохранения
Законы сохранения Кристаллические и аморфные тела в современном мире Выполнила: Попова Людмила Леонасовна, учитель физики МБОУ «СОШ № 14» имени А.М.
Кристаллические и аморфные тела в современном мире Выполнила: Попова Людмила Леонасовна, учитель физики МБОУ «СОШ № 14» имени А.М. Электрическое поле в диэлектриках. Тема 5
Электрическое поле в диэлектриках. Тема 5 Комбинированные задачи. Базовые формулы
Комбинированные задачи. Базовые формулы Ультразвуковой контроль
Ультразвуковой контроль Презентация по физике "Решение задач на движение по реке" - скачать
Презентация по физике "Решение задач на движение по реке" - скачать  Закон всемирного тяготения
Закон всемирного тяготения Движение заряженных частиц в электрическом и магнитном полях
Движение заряженных частиц в электрическом и магнитном полях Герц Генрих Рудольф (1857-1894)
Герц Генрих Рудольф (1857-1894) Оптическая когерентная томография (ОКТ)
Оптическая когерентная томография (ОКТ) Атомно-абсорбционная спектроскопия
Атомно-абсорбционная спектроскопия Прикладная архитектурная акустика
Прикладная архитектурная акустика Мирное применение ядерной энергии
Мирное применение ядерной энергии Электромагнетизм
Электромагнетизм Звуковые волны
Звуковые волны ПАРОВАЯ ТУРБИНА КПД ТЕПЛОВОГО ДВИГАТЕЛЯ
ПАРОВАЯ ТУРБИНА КПД ТЕПЛОВОГО ДВИГАТЕЛЯ Лекция 23 Тема: Электростатическое поле в диэлектрической среде. Поляризованность. Электрическое смещение. Проводники в электр
Лекция 23 Тема: Электростатическое поле в диэлектрической среде. Поляризованность. Электрическое смещение. Проводники в электр Комплексный метод расчета цепей синусоидального тока. Резонанс напряжений
Комплексный метод расчета цепей синусоидального тока. Резонанс напряжений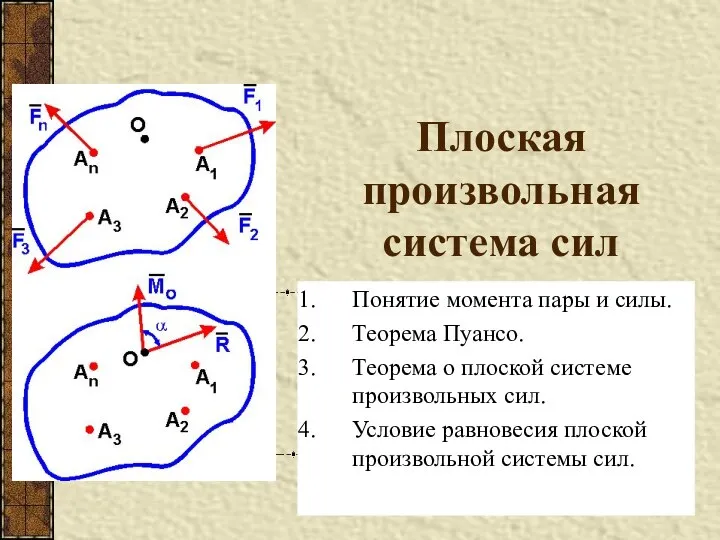 Плоская произвольная система сил
Плоская произвольная система сил Аномальные свойства воды
Аномальные свойства воды