Содержание
- 2. LESSON OBJECTIVES: Concept of electrolytes Define electrolyte, electrolytic solution, ion, cation, anion Arrhenius theory of electrolytic
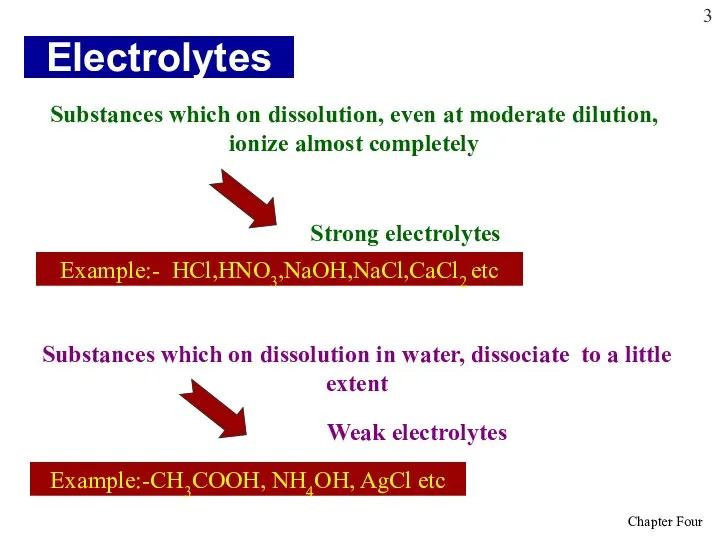
- 3. Electrolytes Substances which on dissolution, even at moderate dilution, ionize almost completely Substances which on dissolution
- 4. In the world of chemistry, an electrolyte is a substance having the free ions so that
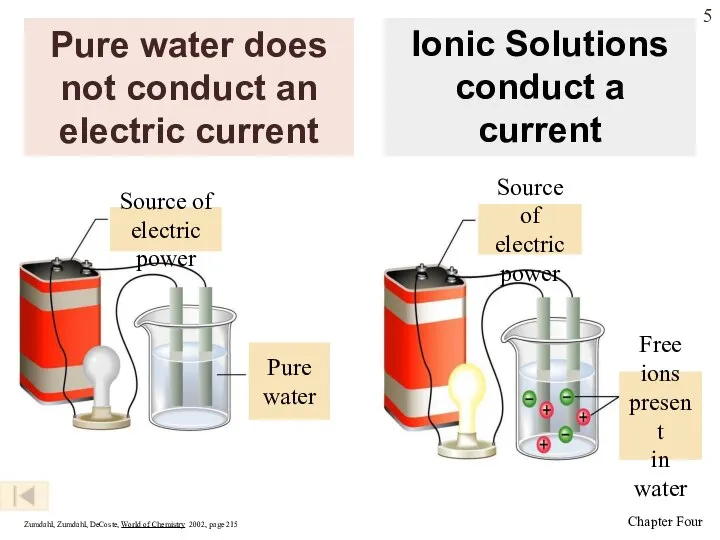
- 5. Pure water does not conduct an electric current Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page
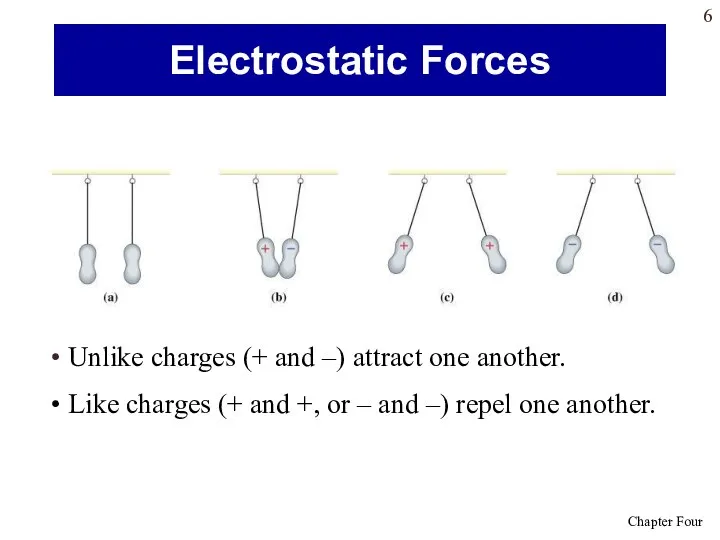
- 6. Unlike charges (+ and –) attract one another. Like charges (+ and +, or – and
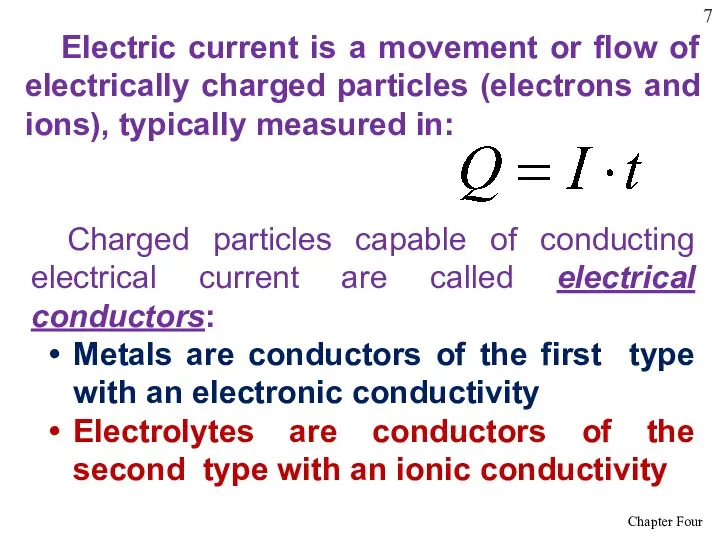
- 7. Electric current is a movement or flow of electrically charged particles (electrons and ions), typically measured
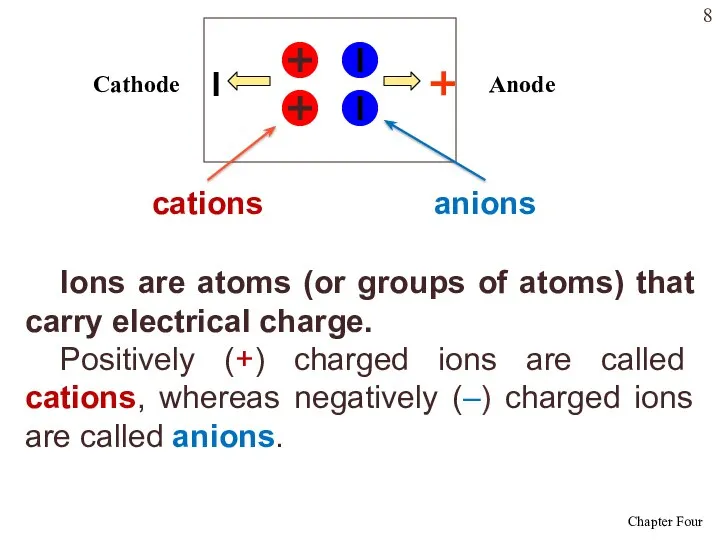
- 8. Ions are atoms (or groups of atoms) that carry electrical charge. Positively (+) charged ions are
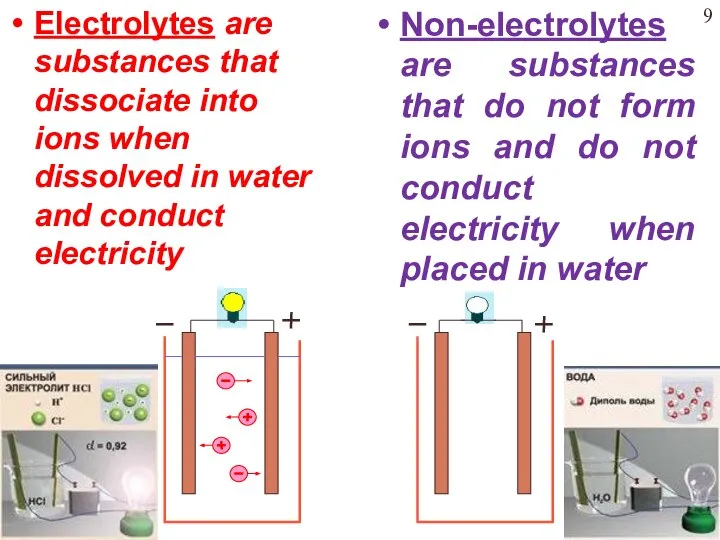
- 9. Electrolytes are substances that dissociate into ions when dissolved in water and conduct electricity Non-electrolytes are
- 10. Electrical Conductivity of Ionic Solutions
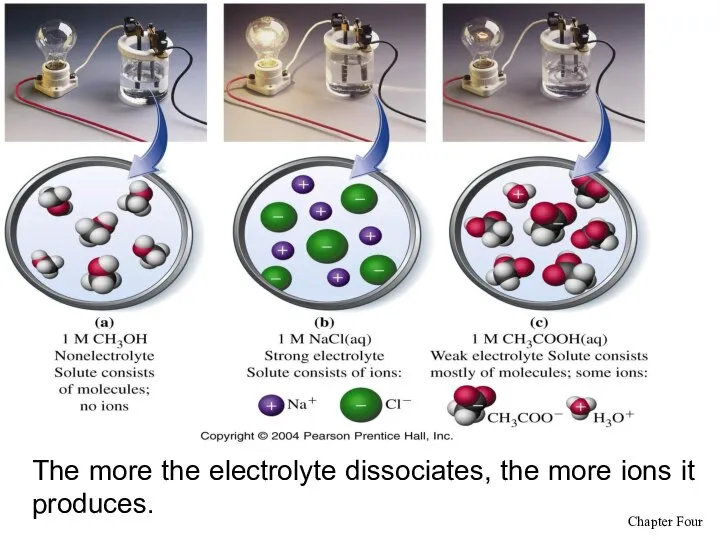
- 11. The more the electrolyte dissociates, the more ions it produces.
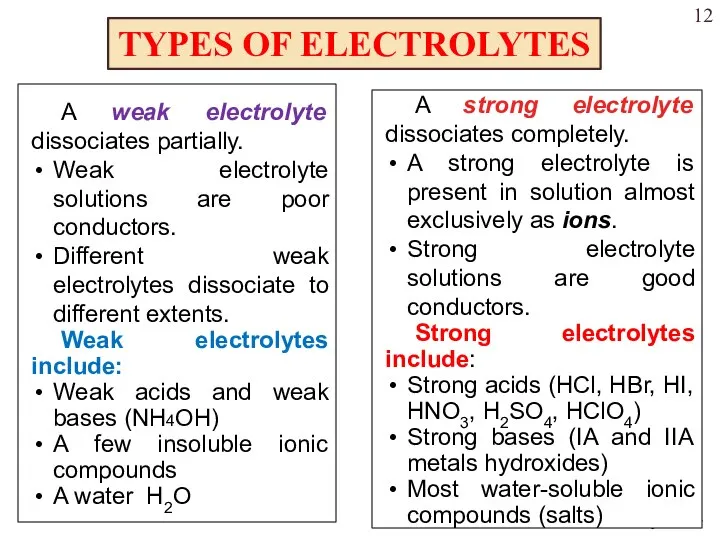
- 12. TYPES OF ELECTROLYTES A weak electrolyte dissociates partially. Weak electrolyte solutions are poor conductors. Different weak
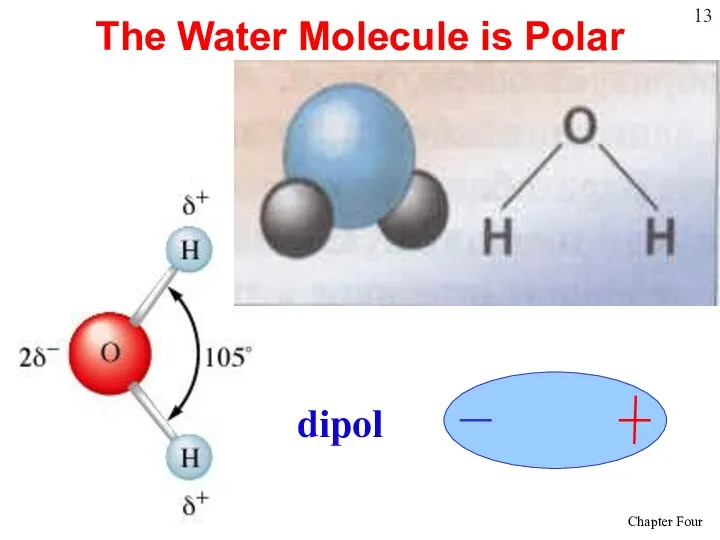
- 13. The Water Molecule is Polar dipol
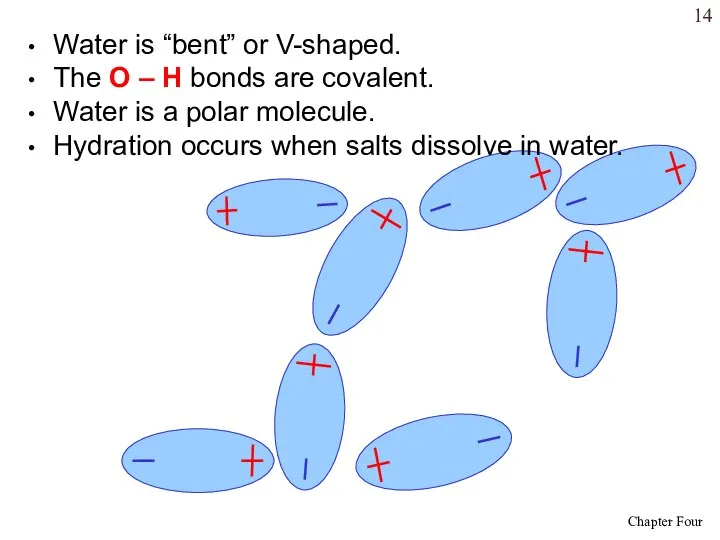
- 14. Water is “bent” or V-shaped. The O – H bonds are covalent. Water is a polar
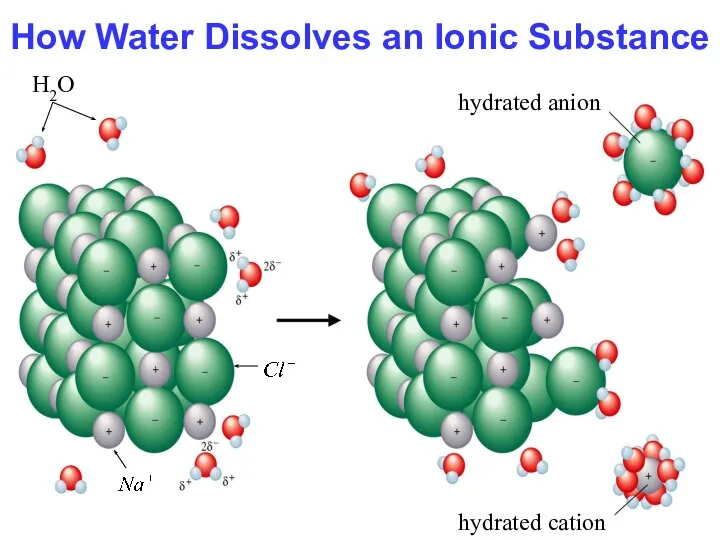
- 15. How Water Dissolves an Ionic Substance H2O hydrated cation hydrated anion
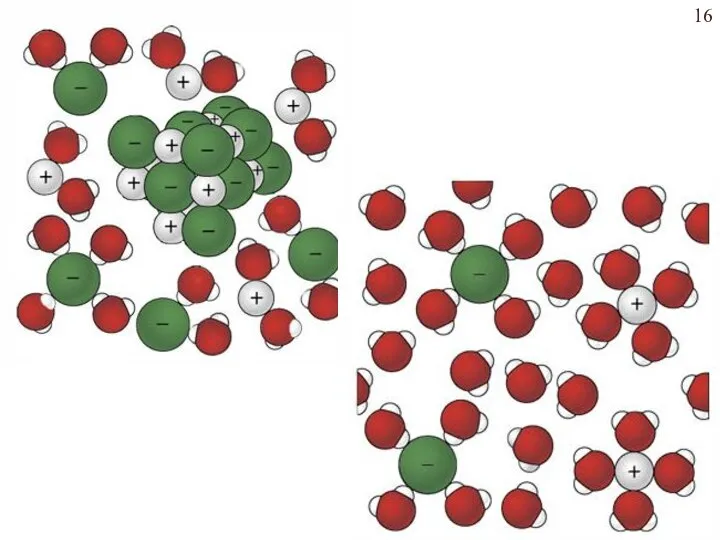
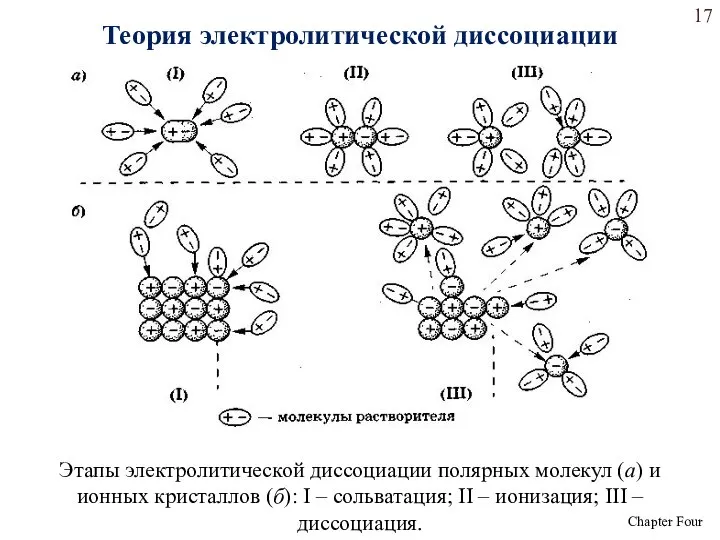
- 17. Этапы электролитической диссоциации полярных молекул (а) и ионных кристаллов (б): I – сольватация; II – ионизация;
- 18. In order to explain the properties of electrolytic solutions, Arrhenius put forth, in 1884, a comprehensive
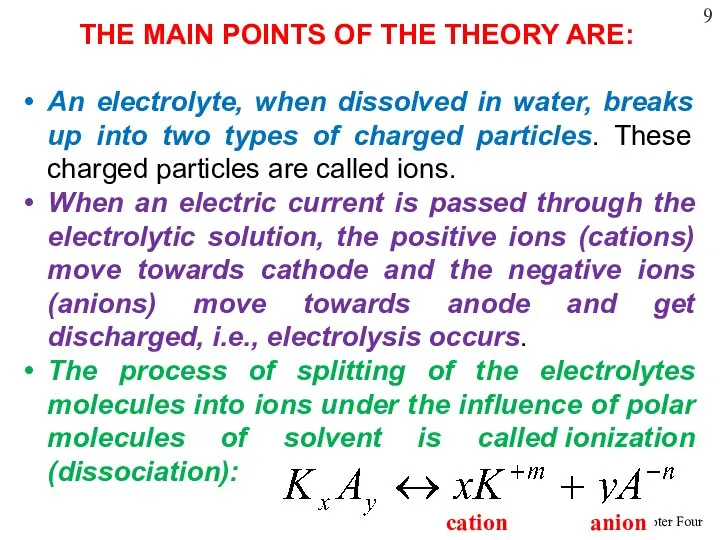
- 19. THE MAIN POINTS OF THE THEORY ARE: An electrolyte, when dissolved in water, breaks up into
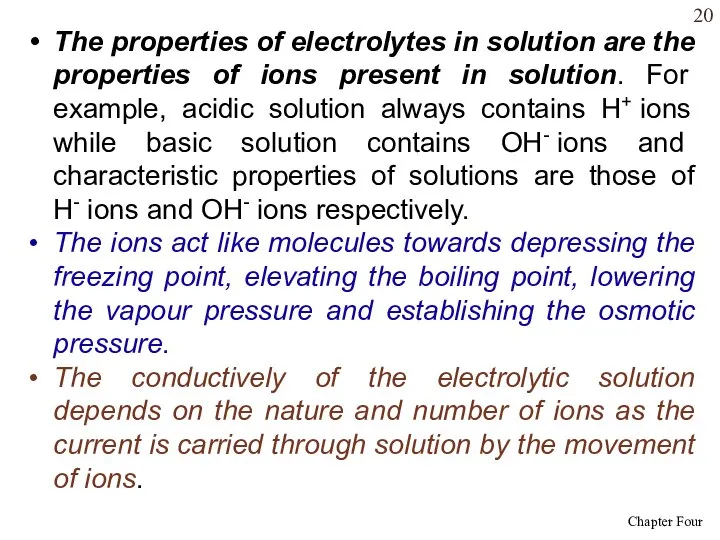
- 20. The properties of electrolytes in solution are the properties of ions present in solution. For example,
- 21. An acid is a substance that increase H+ when dissolved in water: Some acids have more
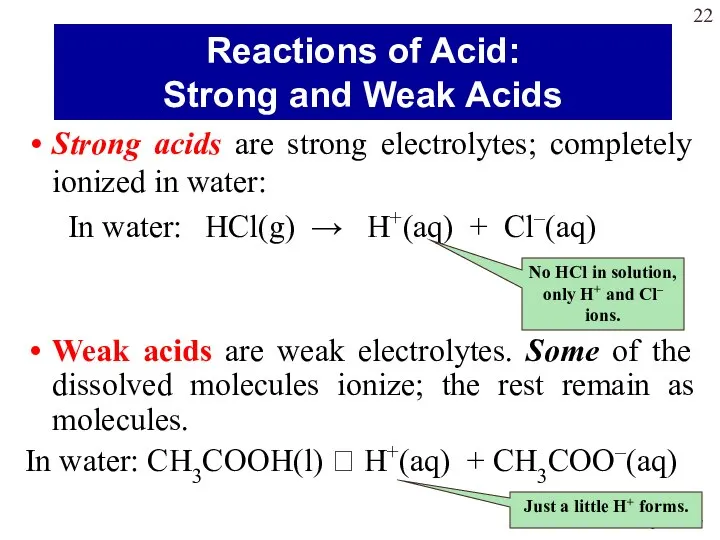
- 22. Strong acids are strong electrolytes; completely ionized in water: In water: HCl(g) → H+(aq) + Cl–(aq)
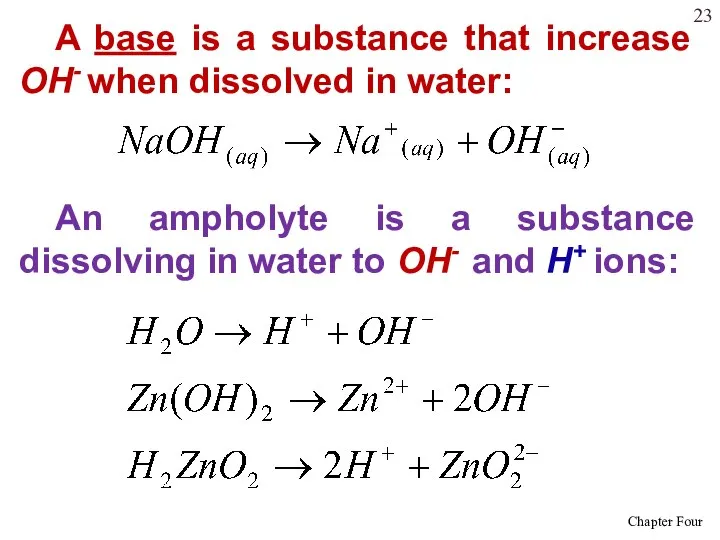
- 23. A base is a substance that increase OH- when dissolved in water: An ampholyte is a
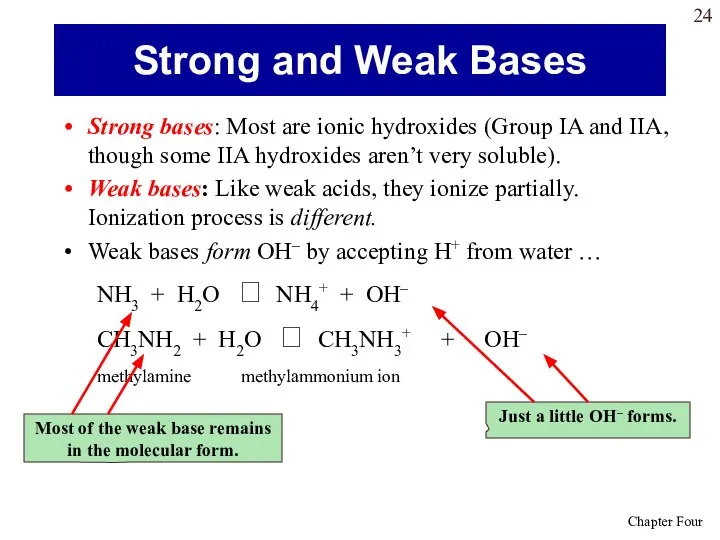
- 24. Strong bases: Most are ionic hydroxides (Group IA and IIA, though some IIA hydroxides aren’t very
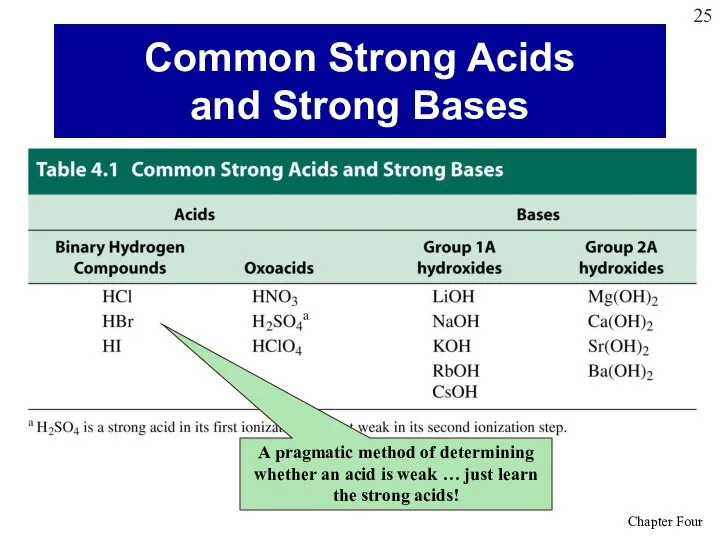
- 25. Common Strong Acids and Strong Bases A pragmatic method of determining whether an acid is weak
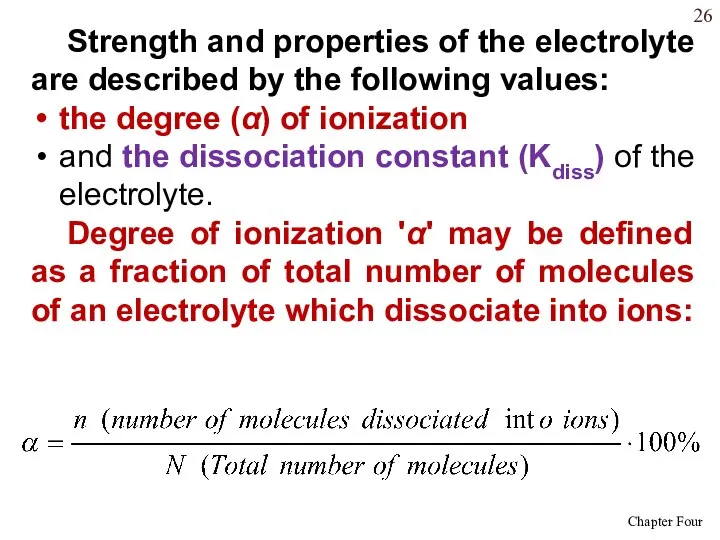
- 26. Strength and properties of the electrolyte are described by the following values: the degree (α) of
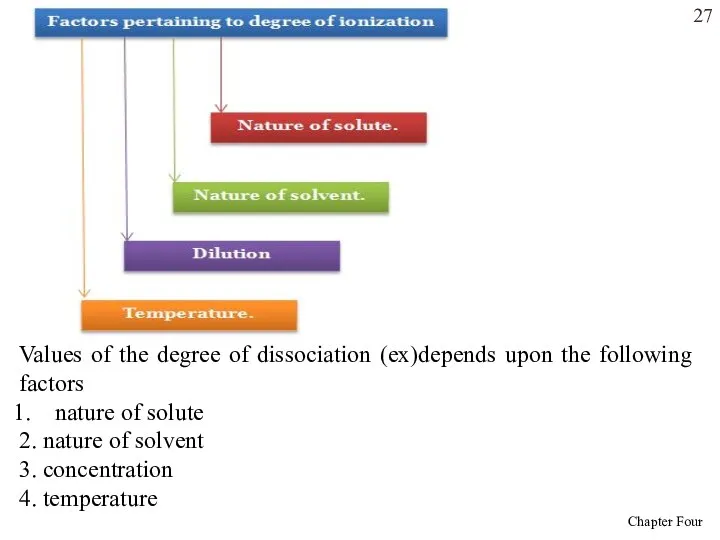
- 27. Values of the degree of dissociation (ex)depends upon the following factors nature of solute 2. nature
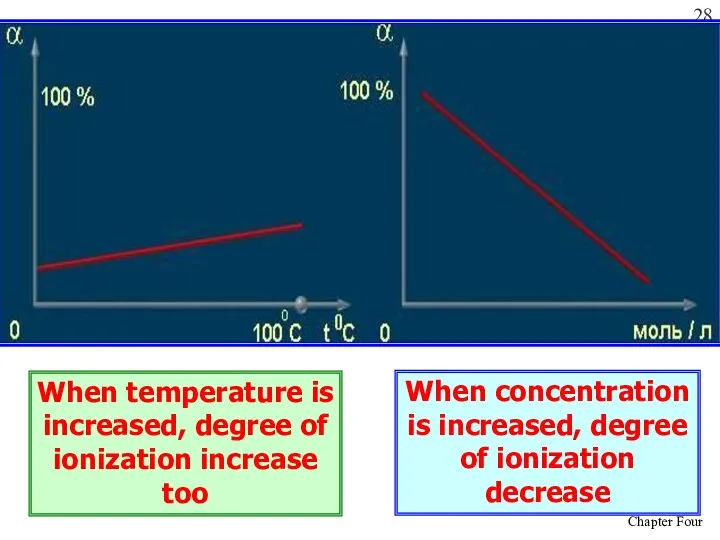
- 28. When temperature is increased, degree of ionization increase too When concentration is increased, degree of ionization
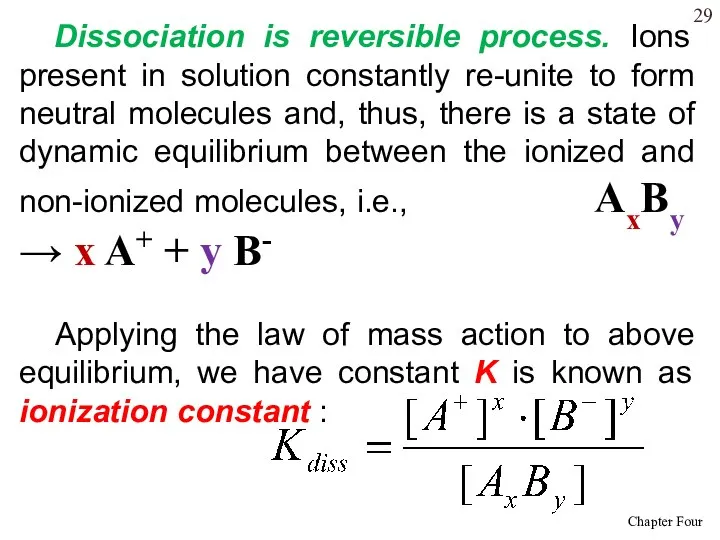
- 29. Dissociation is reversible process. Ions present in solution constantly re-unite to form neutral molecules and, thus,
- 30. For strong electrolytes α>0,3 (30%) and they having high value of Kdiss For weak electrolytes α
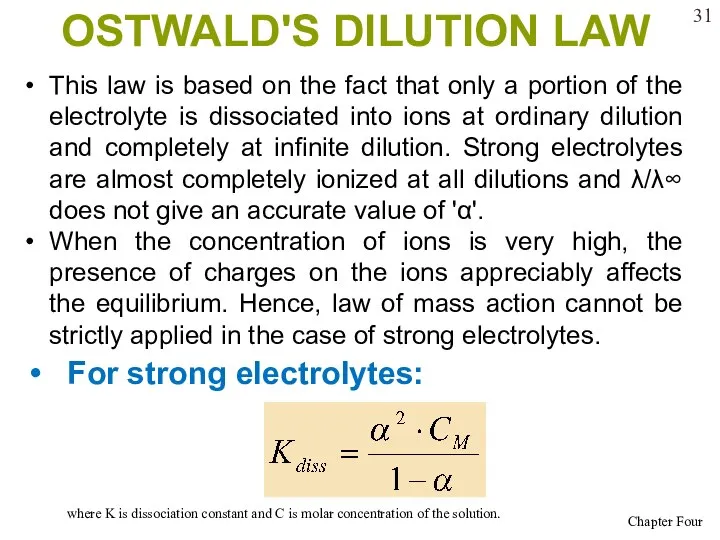
- 31. OSTWALD'S DILUTION LAW For strong electrolytes: where K is dissociation constant and C is molar concentration
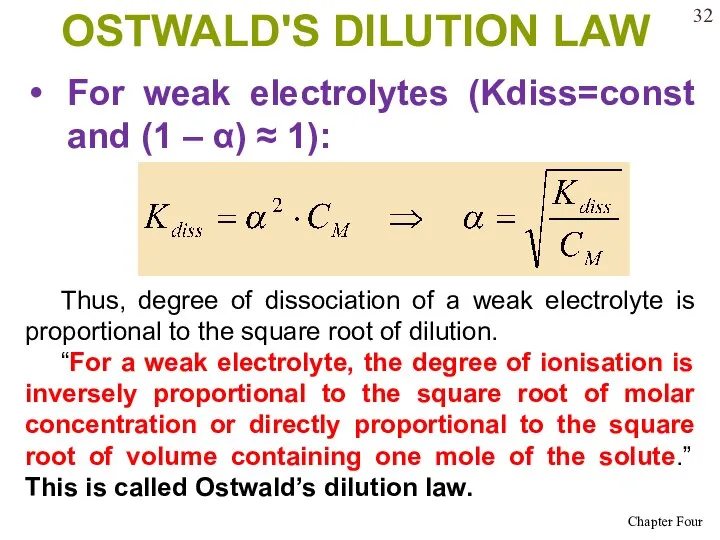
- 32. OSTWALD'S DILUTION LAW For weak electrolytes (Kdiss=const and (1 – α) ≈ 1): Thus, degree of
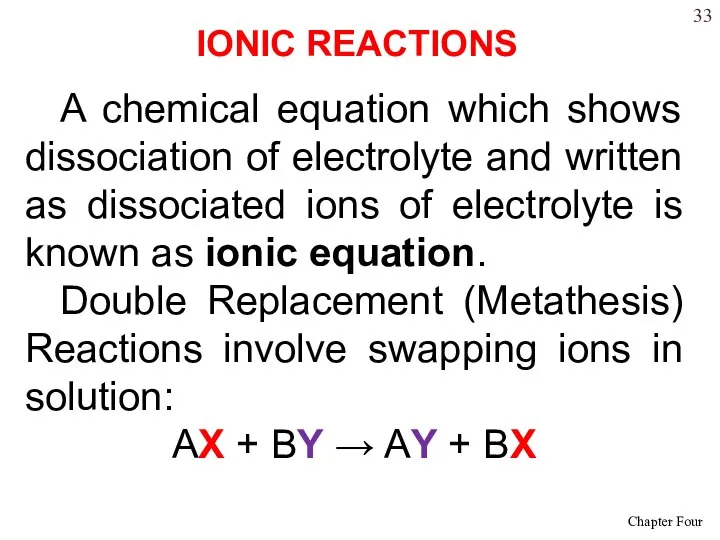
- 33. A chemical equation which shows dissociation of electrolyte and written as dissociated ions of electrolyte is
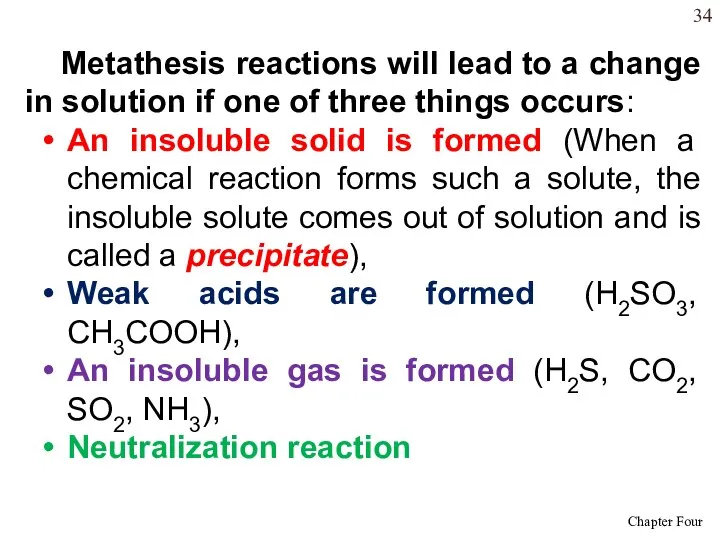
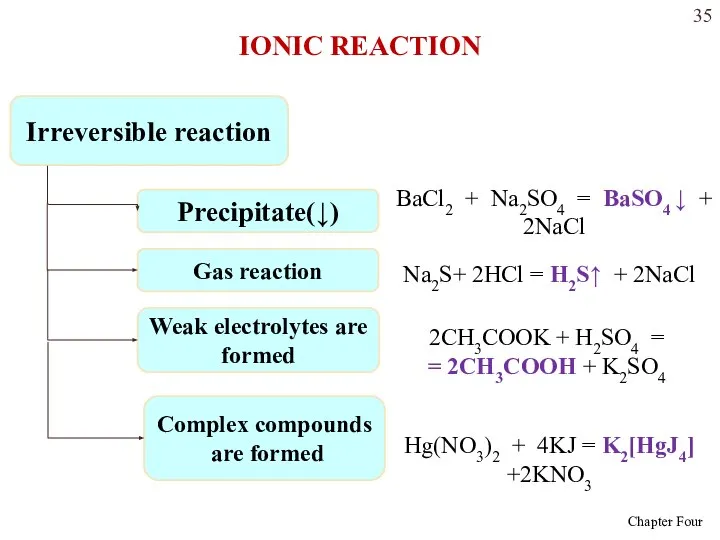
- 34. Metathesis reactions will lead to a change in solution if one of three things occurs: An
- 35. Irreversible reaction Precipitate(↓) BaCl2 + Na2SO4 = BaSO4 ↓ + 2NaCl Gas reaction Na2S+ 2HCl =
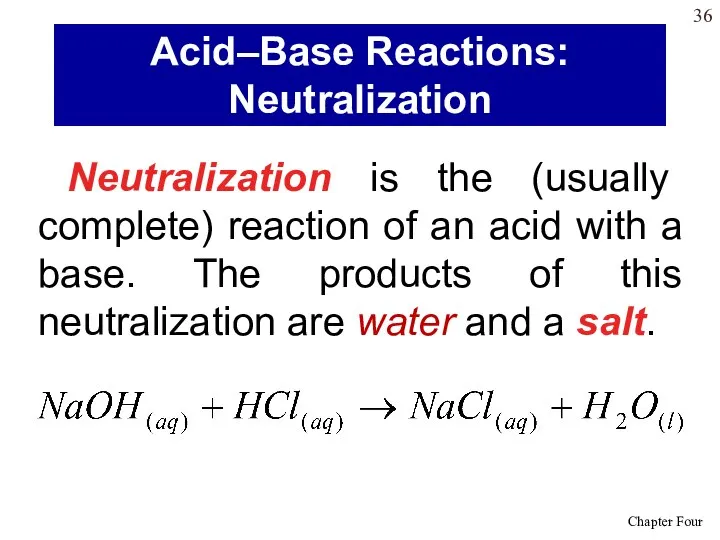
- 36. Neutralization is the (usually complete) reaction of an acid with a base. The products of this
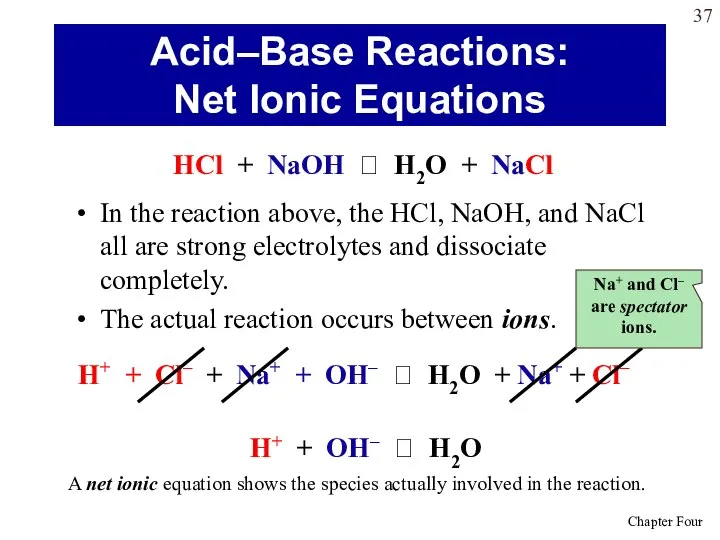
- 37. In the reaction above, the HCl, NaOH, and NaCl all are strong electrolytes and dissociate completely.
- 38. There are limits to the amount of a solute that will dissolve in a given amount
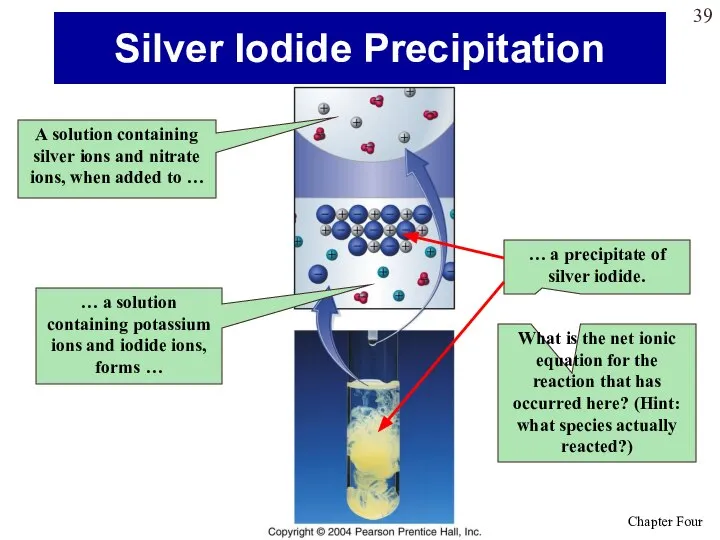
- 39. Silver Iodide Precipitation A solution containing silver ions and nitrate ions, when added to … …
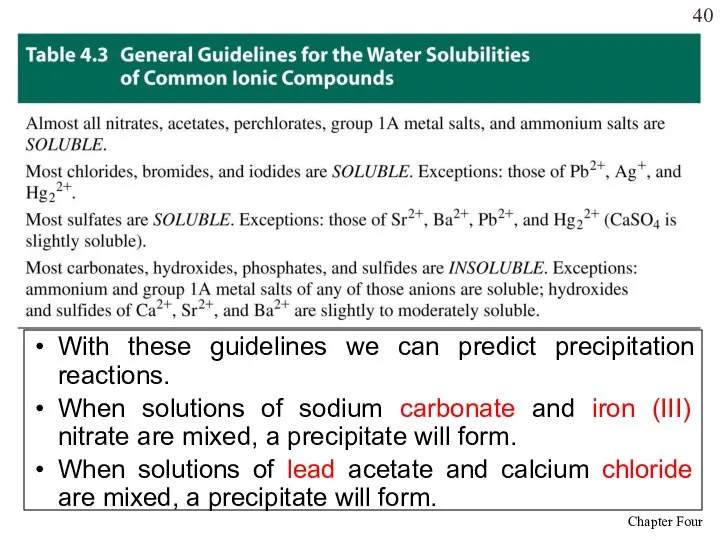
- 40. With these guidelines we can predict precipitation reactions. When solutions of sodium carbonate and iron (III)
- 42. Скачать презентацию





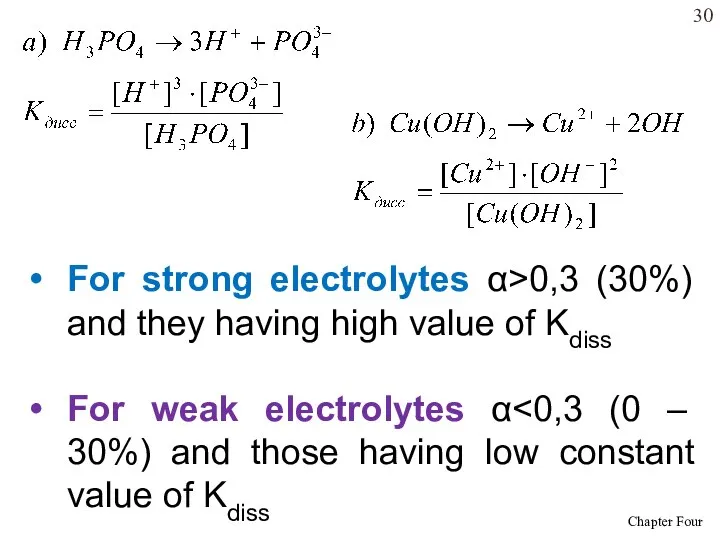
 Йод
Йод Редукторные масла Лукойл
Редукторные масла Лукойл Проект познавательно-исследовательской деятельности. Удивительная соль
Проект познавательно-исследовательской деятельности. Удивительная соль Электрохимические цепи
Электрохимические цепи Презентация по Химии "Синтетические моющие средства." - скачать смотреть бесплатно_
Презентация по Химии "Синтетические моющие средства." - скачать смотреть бесплатно_ Лекарственные растения, содержащие простые фенолы, лигнаны и кумарины
Лекарственные растения, содержащие простые фенолы, лигнаны и кумарины Властивості етанової (оцтової) кислоти
Властивості етанової (оцтової) кислоти ОКИСЛИТЕЛЬНО-ВОССТАНОВИТЕЛЬНЫЕ РЕАКЦИИ 1.ОВР.Классификация ОВР. 2.Метод электронного баланса. 3.Метод полуреакций.
ОКИСЛИТЕЛЬНО-ВОССТАНОВИТЕЛЬНЫЕ РЕАКЦИИ 1.ОВР.Классификация ОВР. 2.Метод электронного баланса. 3.Метод полуреакций.  Миорелаксанты. Общая характеристика
Миорелаксанты. Общая характеристика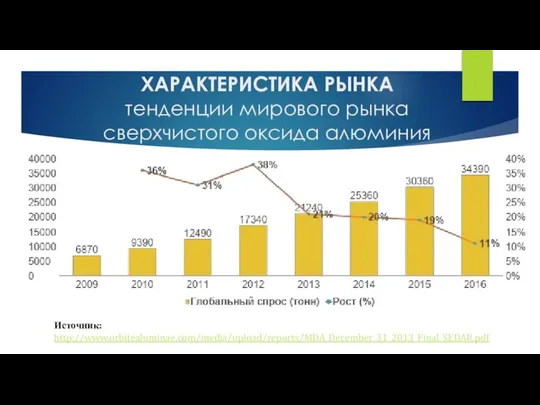 Характеристика рынка. Тенденции мирового рынка сверхчистого оксида алюминия
Характеристика рынка. Тенденции мирового рынка сверхчистого оксида алюминия Качественные реакции. Катионы
Качественные реакции. Катионы Инсектициды на основе бакуловирусов
Инсектициды на основе бакуловирусов Основные требования к химическим реакторам
Основные требования к химическим реакторам Строение вещества. Диффузия. Броуновское движение
Строение вещества. Диффузия. Броуновское движение Презентация по Химии "НИТРАТЫ. ИХ ВЛИЯНИЕ НА ЗДОРОВЬЕ ЛЮДЕЙ" - скачать смотреть бесплатно
Презентация по Химии "НИТРАТЫ. ИХ ВЛИЯНИЕ НА ЗДОРОВЬЕ ЛЮДЕЙ" - скачать смотреть бесплатно Типы окисления. Понятие об антиоксидантной системе
Типы окисления. Понятие об антиоксидантной системе  Важнейшие классы неорганических соединений
Важнейшие классы неорганических соединений Третий закон Менделя. Урок 4
Третий закон Менделя. Урок 4 Металлы. Положение в ПСХЭ Д.И. Менделеева. Физические свойства металлов .
Металлы. Положение в ПСХЭ Д.И. Менделеева. Физические свойства металлов . Презентация по химии Кислоты 8 класс
Презентация по химии Кислоты 8 класс Свойства и применение уксусной кислоты
Свойства и применение уксусной кислоты Химические свойства металлов
Химические свойства металлов Коррозия металлов
Коррозия металлов Трансмиссионные масла
Трансмиссионные масла Берилій
Берилій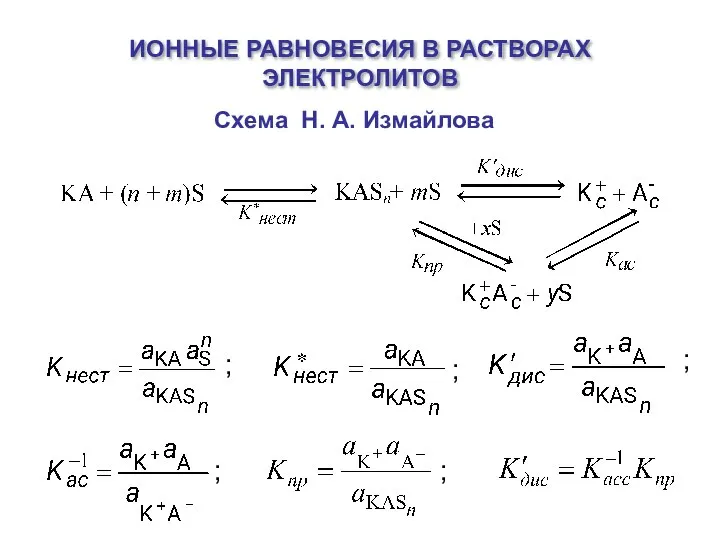 Ионные равновесия в растворах электролитов
Ионные равновесия в растворах электролитов Аттестационная работа. Использование цифровой лаборатории «Архимед» во внеурочной деятельности по химии
Аттестационная работа. Использование цифровой лаборатории «Архимед» во внеурочной деятельности по химии Молекулярное строение твердых тел, жидкостей и газов
Молекулярное строение твердых тел, жидкостей и газов