Содержание
- 2. QUIZ ME NEXT 1 What is it a true solution? a pure substances in water compounds
- 3. QUIZ ME NEXT 2 The amount of a solute dissolved in a given amount of solvent
- 4. QUIZ ME 3 What is the molar concentration of solution? the number of equivalent-moles of solute
- 5. QUIZ ME 4 What is the molality of solution? the number of equivalent-moles of solute per
- 6. Nature of Solute Non-electrolytic are substances that do not form ions and do not conduct electricity
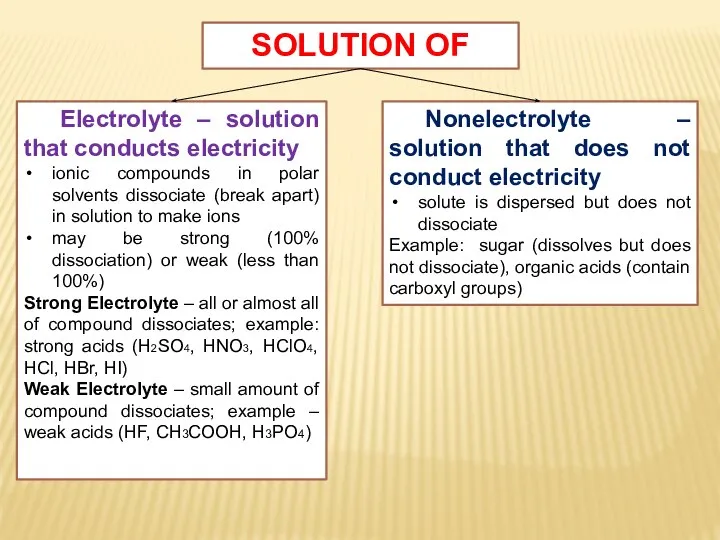
- 7. SOLUTION OF Electrolyte – solution that conducts electricity ionic compounds in polar solvents dissociate (break apart)
- 8. COLLIGATIVE PROPERTIES Colligative properties are the set of properties that depend only on the concentration of
- 9. 1) Vapor Pressure Lowering (1st Raoult’s Law) Related to boiling point 2) Freezing Point Depression Salt
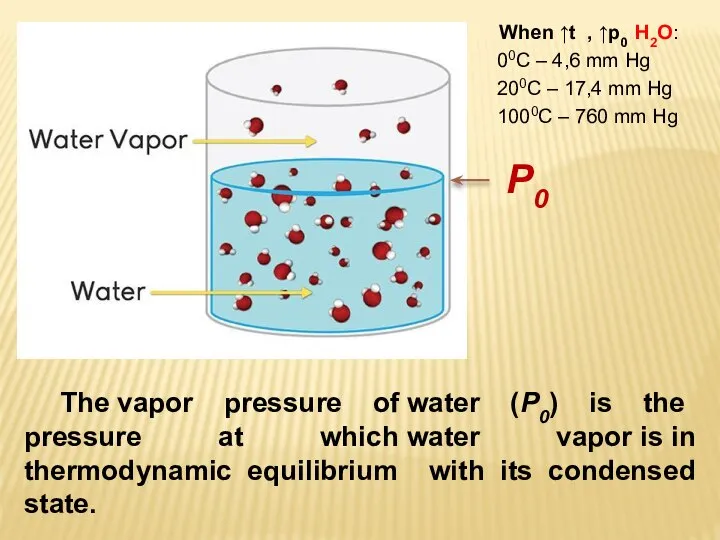
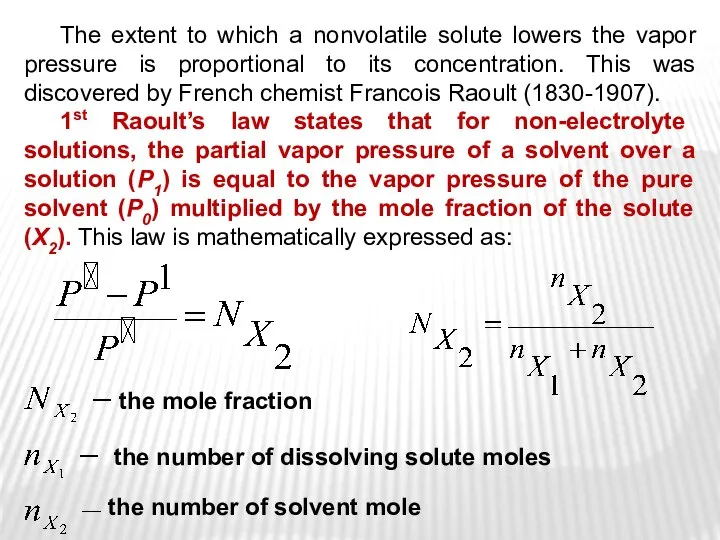
- 10. The vapor pressure of water (P0) is the pressure at which water vapor is in thermodynamic
- 11. Vapor pressure depends on various factors: the nature of the liquid, the presence of dissolved substances
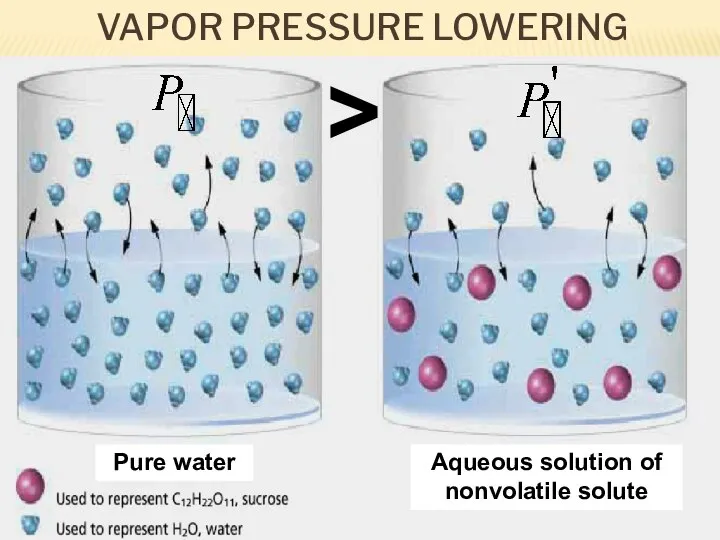
- 12. VAPOR PRESSURE LOWERING Pure water Aqueous solution of nonvolatile solute >
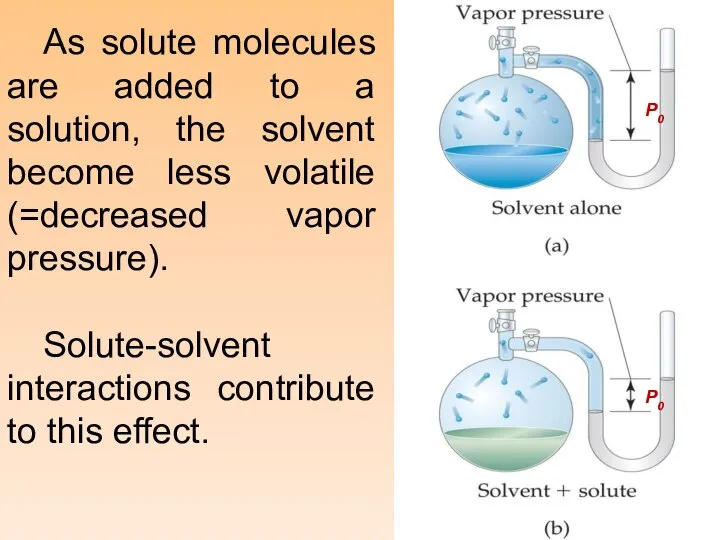
- 13. As solute molecules are added to a solution, the solvent become less volatile (=decreased vapor pressure).
- 14. The extent to which a nonvolatile solute lowers the vapor pressure is proportional to its concentration.
- 15. The temperature at which vapor pressure is equal to the atmospheric pressure (p0= pаtm) is called
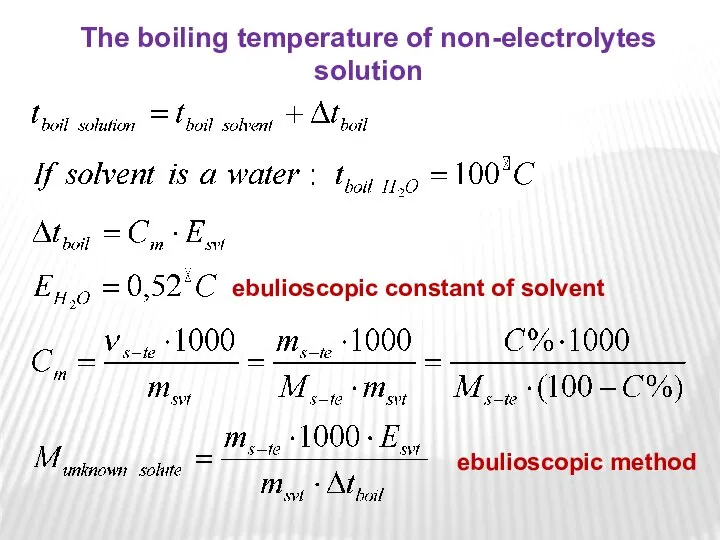
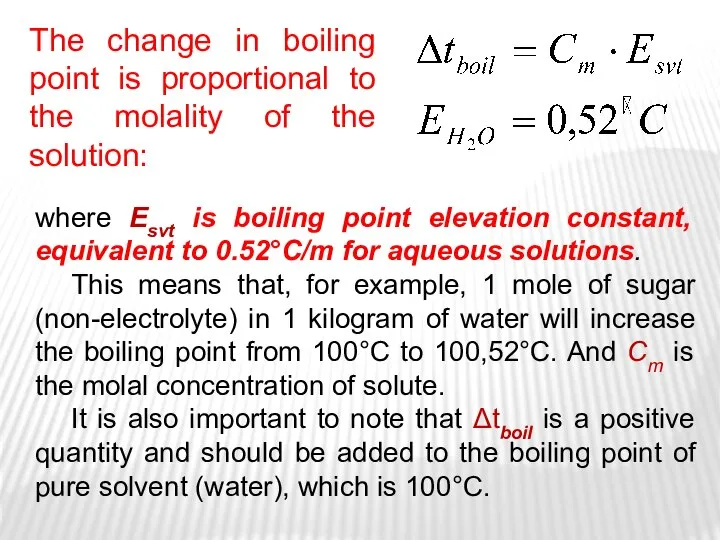
- 16. The 2nd Raoult’s Law One consequence of Raoult's law is that the boiling point of a
- 17. ebulioscopic constant of solvent The boiling temperature of non-electrolytes solution ebulioscopic method
- 18. where Esvt is boiling point elevation constant, equivalent to 0.52°C/m for aqueous solutions. This means that,
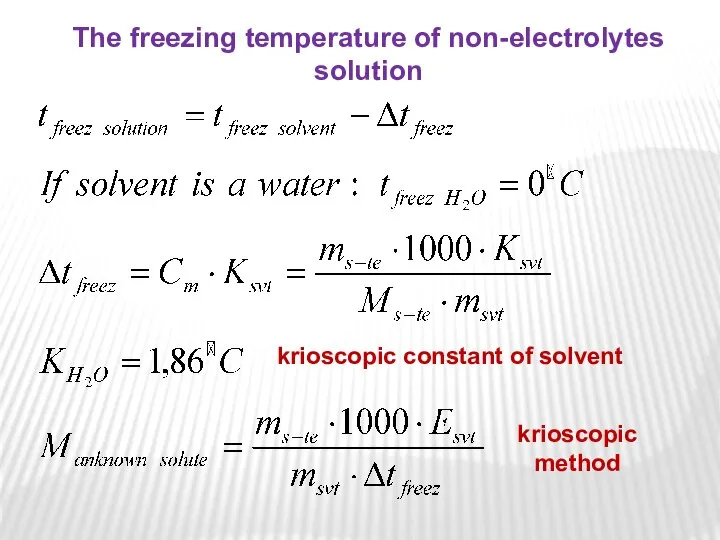
- 19. krioscopic constant of solvent The freezing temperature of non-electrolytes solution krioscopic method
- 20. where Ksvt is the freezing point depression constant equivalent to -1,86°C/m for aqueous solutions. Again, for
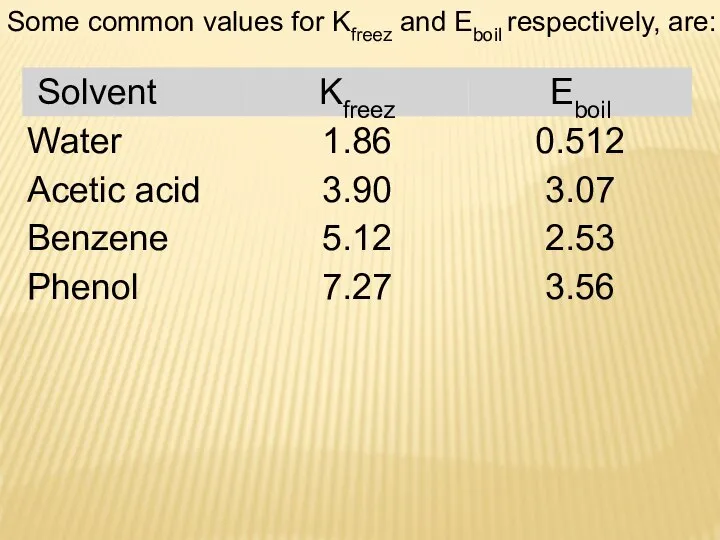
- 21. Some common values for Kfreez and Eboil respectively, are:
- 22. In 1784, the French physicist Jean Antoine Nollet discovered that a pig's bladder filled with a
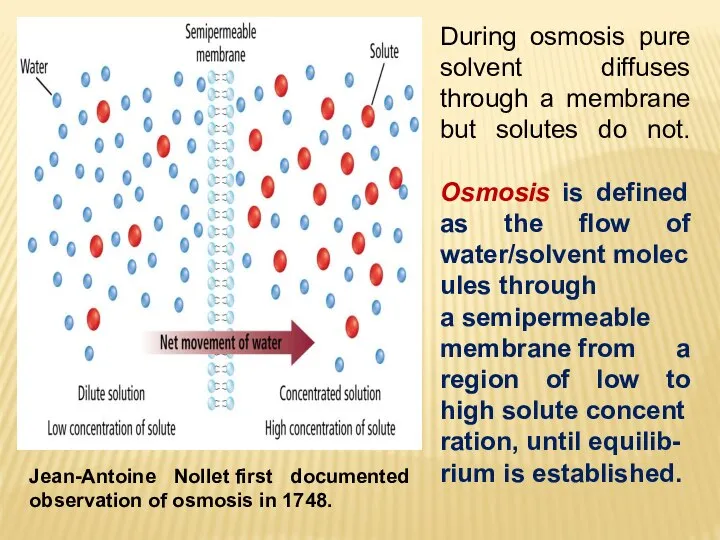
- 23. Jean-Antoine Nollet first documented observation of osmosis in 1748. During osmosis pure solvent diffuses through a
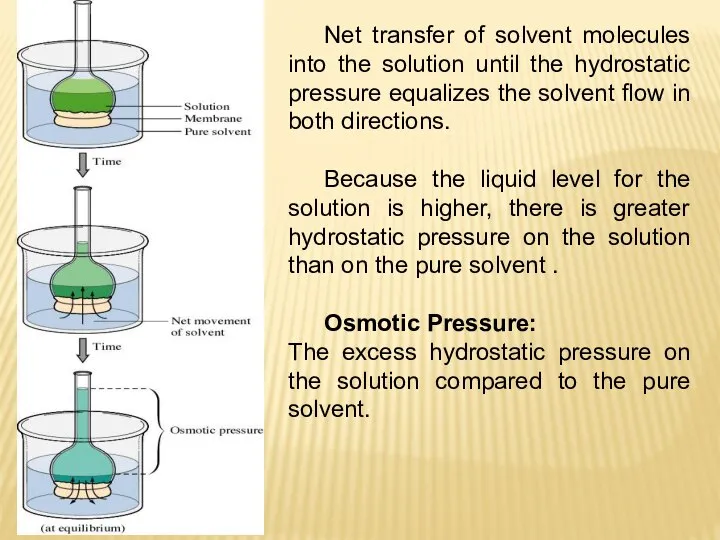
- 24. Net transfer of solvent molecules into the solution until the hydrostatic pressure equalizes the solvent flow
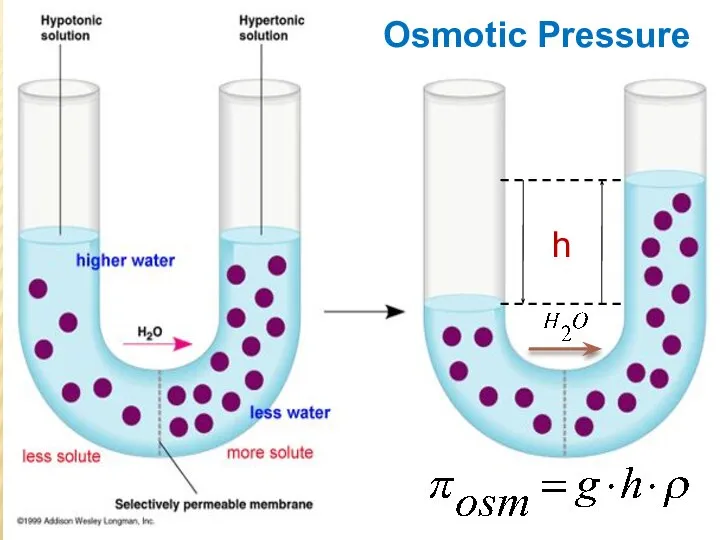
- 25. Osmotic Pressure h
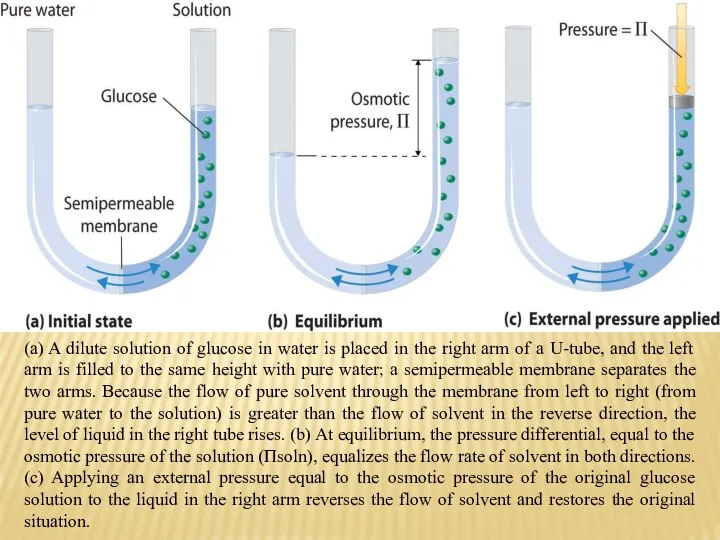
- 26. (a) A dilute solution of glucose in water is placed in the right arm of a
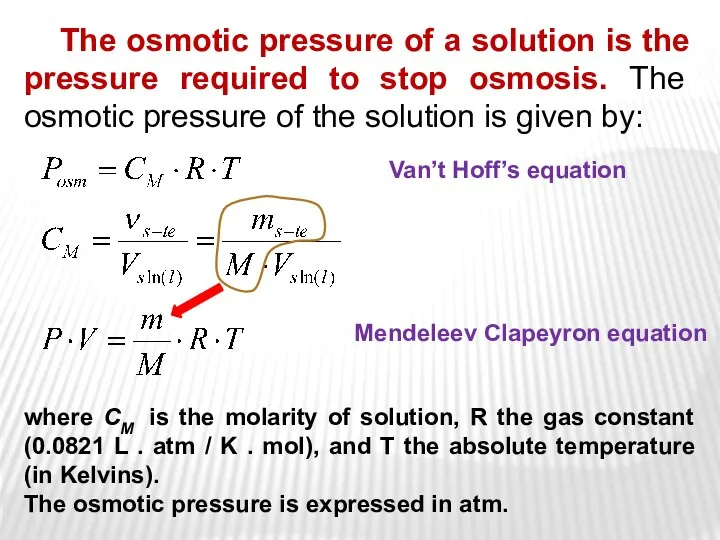
- 27. The osmotic pressure of a solution is the pressure required to stop osmosis. The osmotic pressure
- 28. Molarirty units are most appropriate in calculating which of the following? QUIZ ME A) freezingpoint depression
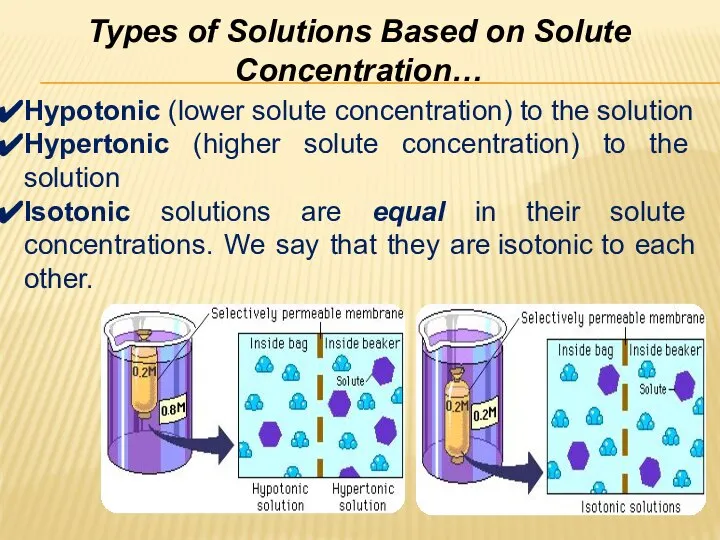
- 29. Types of Solutions Based on Solute Concentration… Hypotonic (lower solute concentration) to the solution Hypertonic (higher
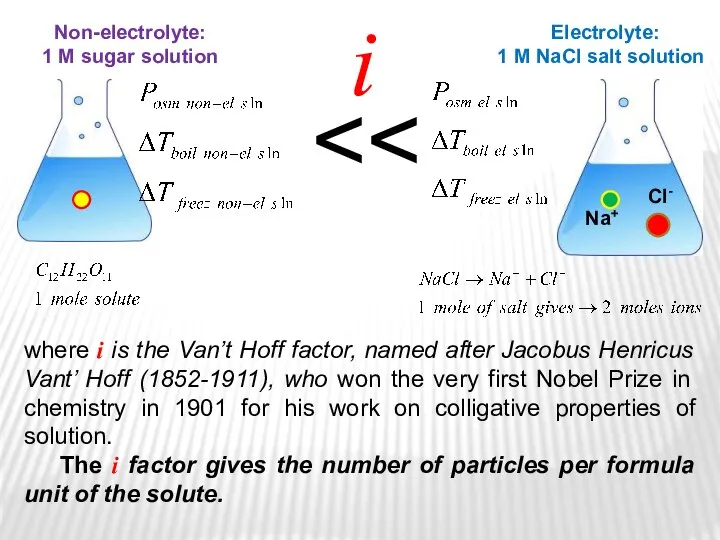
- 30. Non-electrolyte: 1 М sugar solution Electrolyte: 1 М NaCl salt solution where i is the Van’t
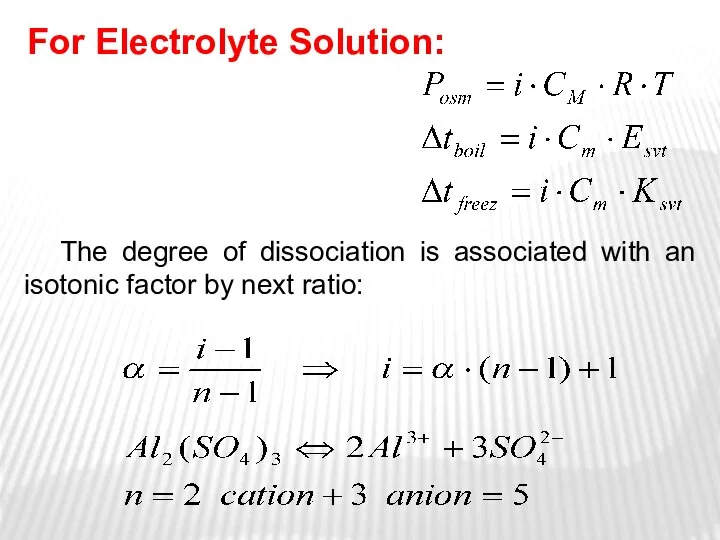
- 31. The degree of dissociation is associated with an isotonic factor by next ratio: For Electrolyte Solution:
- 32. If equal numbers of moles of each of the following are dissolved in 1 kg of
- 33. SUMMARY OF FACTS AND CONCEPTS 1. Colligative properties (or collective properties) are properties that depend only
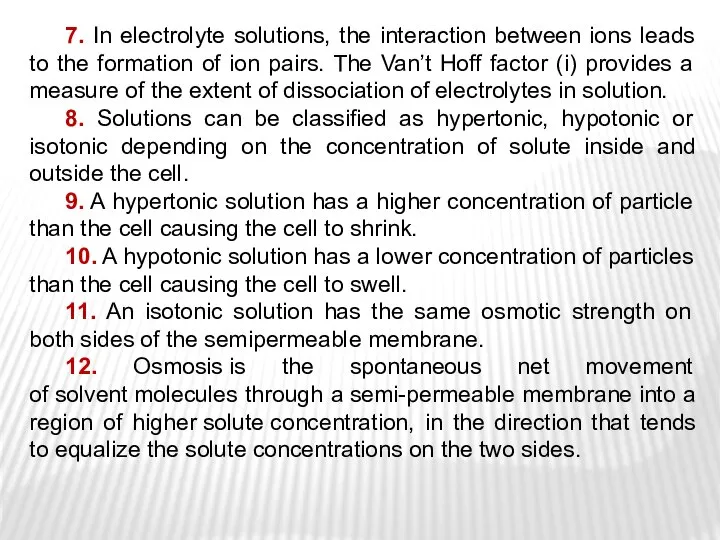
- 34. 7. In electrolyte solutions, the interaction between ions leads to the formation of ion pairs. The
- 36. Скачать презентацию















 Соли. Состав и номенклатура
Соли. Состав и номенклатура Аттестационная работа. Проектно - исследовательская технология, как способ формирования УУД по химии
Аттестационная работа. Проектно - исследовательская технология, как способ формирования УУД по химии студентка группы ГЭ-1-07 факультета ЭиУ Малышева Е.И. «Биомасса – альтернативный источник энергии» студентка группы ГЭ-1-07 фак
студентка группы ГЭ-1-07 факультета ЭиУ Малышева Е.И. «Биомасса – альтернативный источник энергии» студентка группы ГЭ-1-07 фак Углеводы
Углеводы Простые вещества металлы
Простые вещества металлы Уроки зельеварения. Задача 6
Уроки зельеварения. Задача 6 Матчворк Підготувала учениця 11-А класу Шведюк Людмила
Матчворк Підготувала учениця 11-А класу Шведюк Людмила  Уран. Получение урана
Уран. Получение урана Азот
Азот Сера
Сера В чем секрет любви к мороженому ?
В чем секрет любви к мороженому ? Литье под давлением реактопластов
Литье под давлением реактопластов Олигомеризация олефинов
Олигомеризация олефинов Кристаллография. Точечные группы симметрии, принцип их вывода с помощью понятия о группах. Формы кристаллов низшей категории
Кристаллография. Точечные группы симметрии, принцип их вывода с помощью понятия о группах. Формы кристаллов низшей категории Теоретические основы технологии неорганических веществ. (Тема 2)
Теоретические основы технологии неорганических веществ. (Тема 2) Химия биогенных элементов p -блока
Химия биогенных элементов p -блока Характеристика химического элемента по кислотно-основным свойствам образуемых им соединений. Амфотерные оксиды и гидроксиды
Характеристика химического элемента по кислотно-основным свойствам образуемых им соединений. Амфотерные оксиды и гидроксиды Азотная кислота – HNO3 – «взрывоопасная царская особа» МБОУ «Рождественская средняя общеобразовательная школа» Собинско
Азотная кислота – HNO3 – «взрывоопасная царская особа» МБОУ «Рождественская средняя общеобразовательная школа» Собинско Высокомолекулярные вещества
Высокомолекулярные вещества Фосфор и его соединения
Фосфор и его соединения Арилалкиламины, гидроксифенилалкиламины и их производные
Арилалкиламины, гидроксифенилалкиламины и их производные Амфотерні гідроксиди
Амфотерні гідроксиди Презентация по Химии "Природні І синтетичні Органічні речовини" - скачать смотреть бесплатно
Презентация по Химии "Природні І синтетичні Органічні речовини" - скачать смотреть бесплатно Эндогенная серия. Карбонатитовая группа
Эндогенная серия. Карбонатитовая группа Поверхностные явления
Поверхностные явления Коррозия металлов
Коррозия металлов Химиялық, принципиалдық және технологиялық сызбанұсқалар. Технологиялқ процестерді ұйымдастыру принциптері
Химиялық, принципиалдық және технологиялық сызбанұсқалар. Технологиялқ процестерді ұйымдастыру принциптері Реактор получения элементарной серы
Реактор получения элементарной серы