Содержание
- 2. LESSON OBJECTIVES: Ionic product of water. Notion of pH Be able to calculate pH and pOH
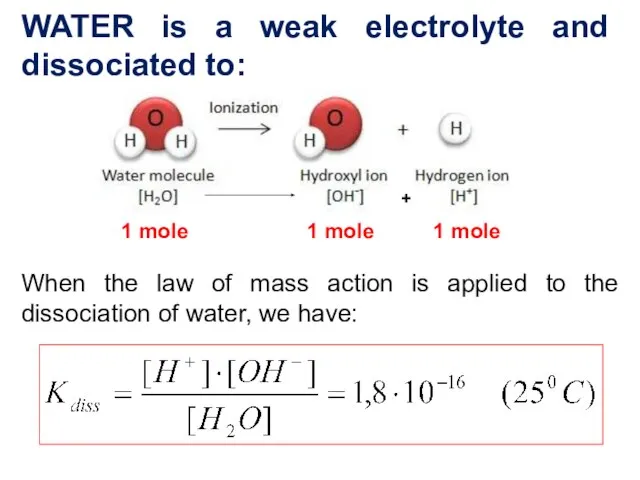
- 3. WATER is a weak electrolyte and dissociated to: When the law of mass action is applied
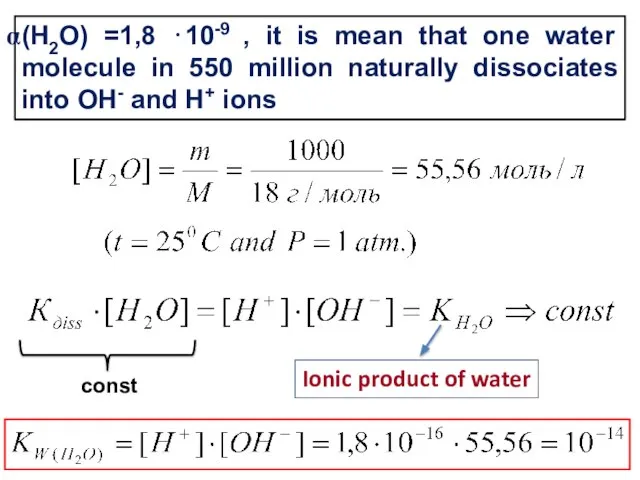
- 4. (Н2О) =1,8 ⋅10-9 , it is mean that one water molecule in 550 million naturally dissociates
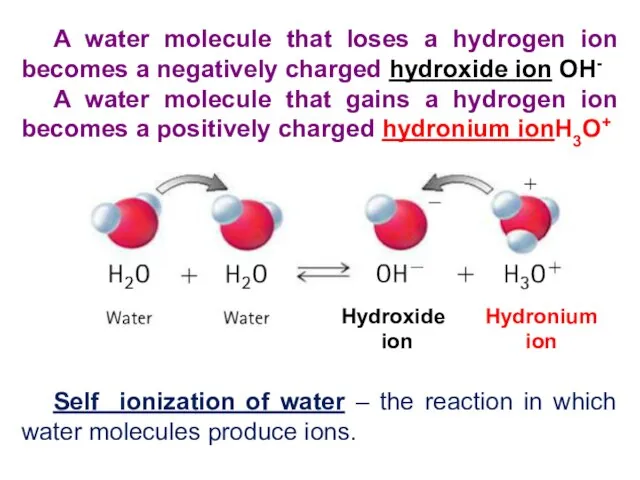
- 5. A water molecule that loses a hydrogen ion becomes a negatively charged hydroxide ion OH- A
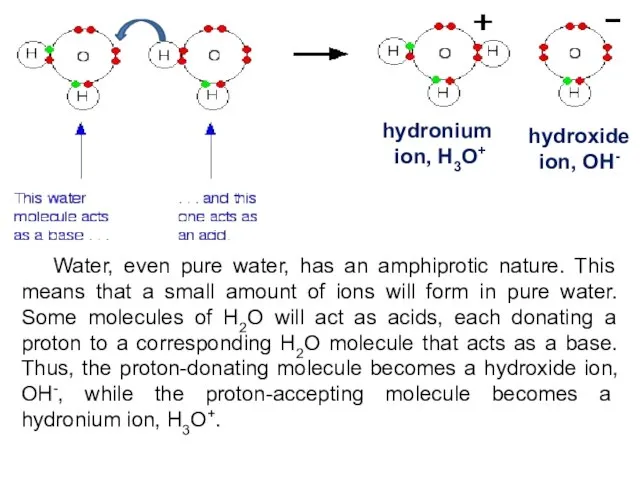
- 6. Water, even pure water, has an amphiprotic nature. This means that a small amount of ions
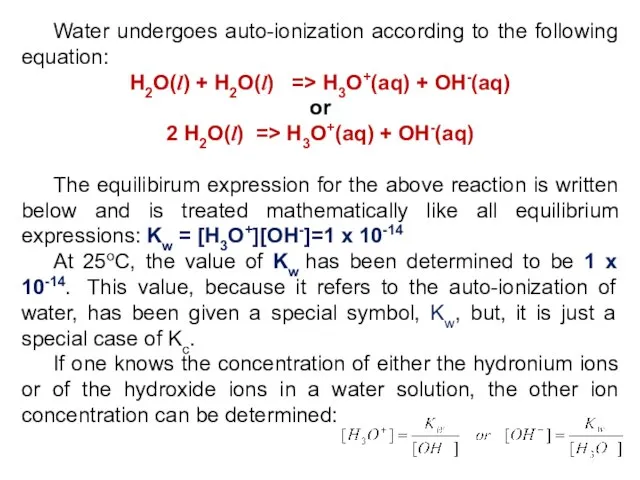
- 7. Water undergoes auto-ionization according to the following equation: H2O(l) + H2O(l) => H3O+(aq) + OH-(aq) or
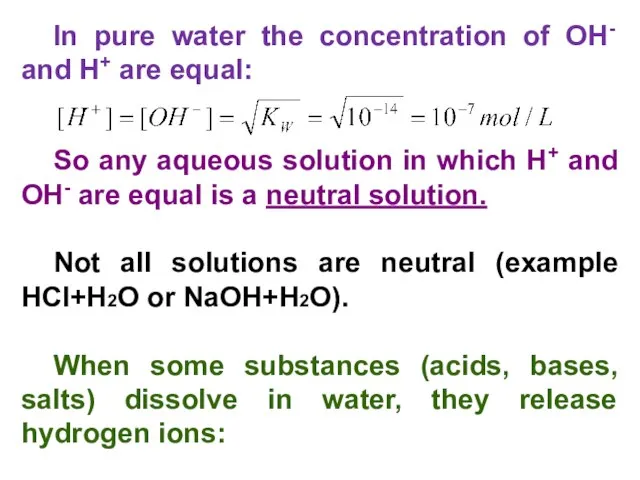
- 8. In pure water the concentration of OH- and H+ are equal: So any aqueous solution in
- 9. When hydrogen chloride dissolves in water, it forms hydrogen-ions: In the previous HCl solution – acidic
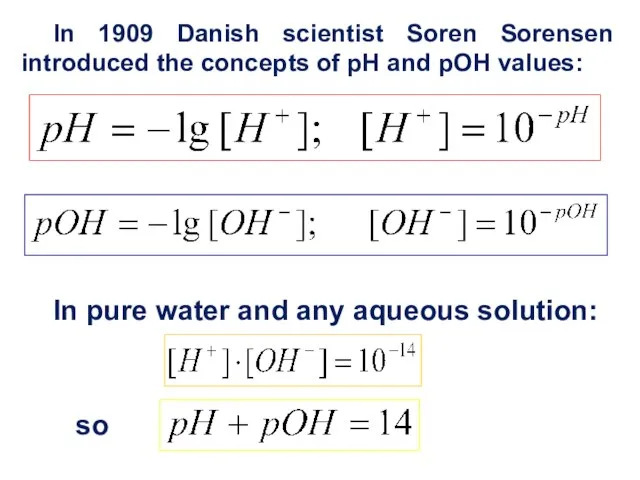
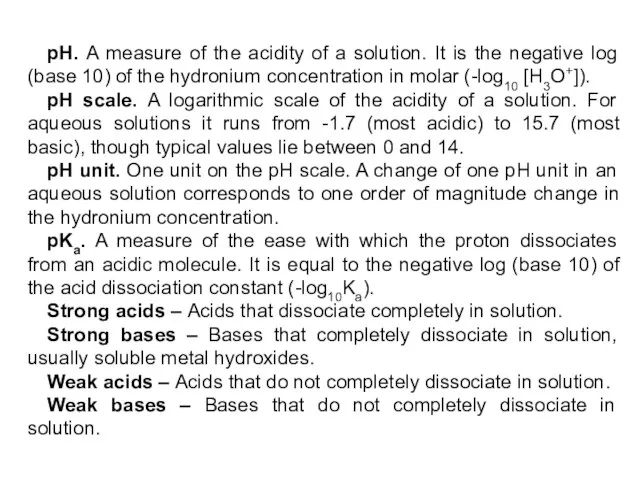
- 10. In pure water and any aqueous solution: so In 1909 Danish scientist Soren Sorensen introduced the
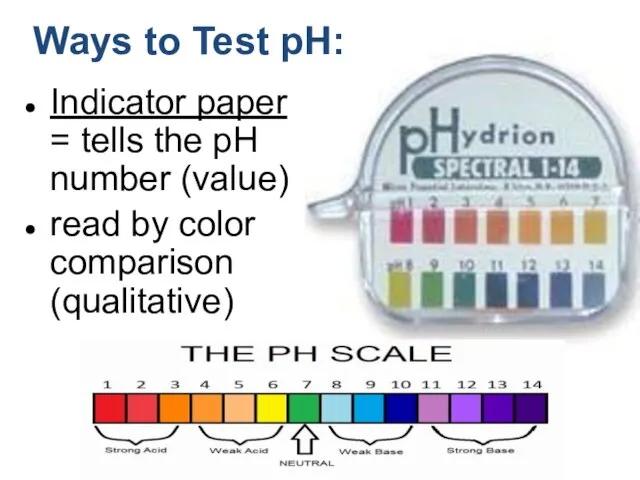
- 11. The pH scale is used to express [H+]
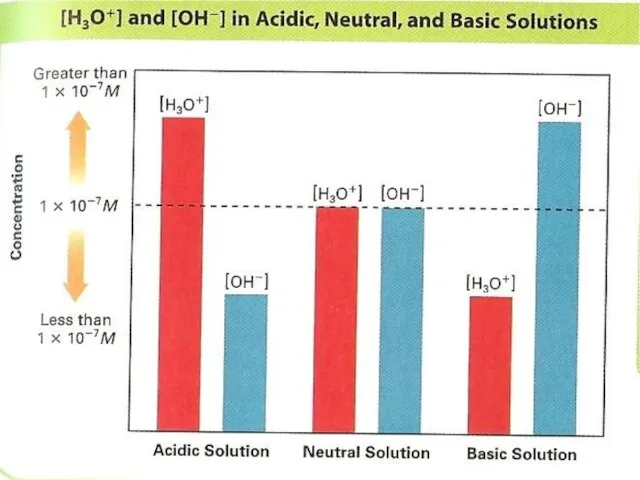
- 12. Classifying Solutions A solution in which [H+] is greater than 1 x 10-7 has a pH
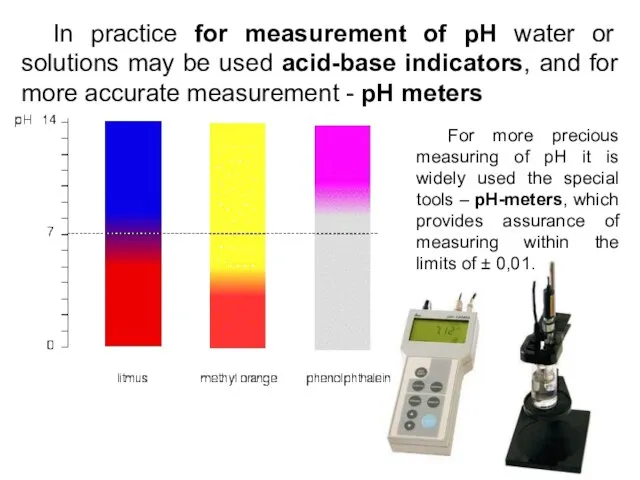
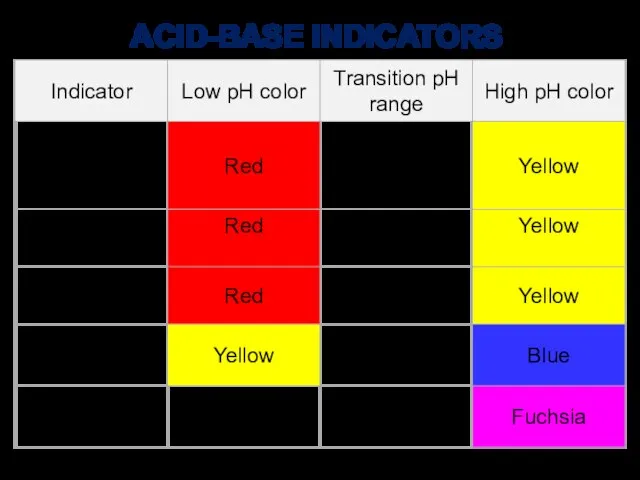
- 14. In practice for measurement of pH water or solutions may be used acid-base indicators, and for
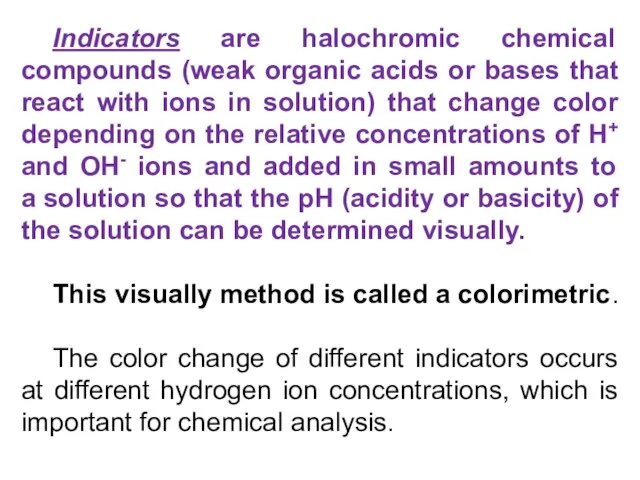
- 15. Indicators are halochromic chemical compounds (weak organic acids or bases that react with ions in solution)
- 16. A Universal indicator is a pH indicator composed of a blend of several compounds that exhibits
- 17. Indicator paper = tells the pH number (value) read by color comparison (qualitative) Ways to Test
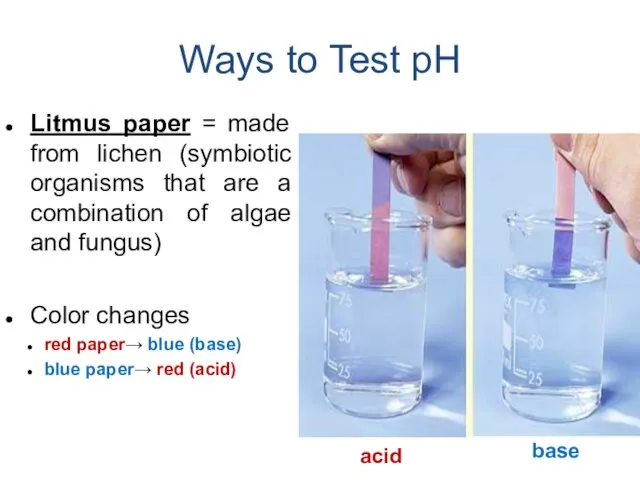
- 18. Ways to Test pH Litmus paper = made from lichen (symbiotic organisms that are a combination
- 19. ACID-BASE INDICATORS
- 20. pH meter Measures amount of H+ ions in the solution Digital readout Most accurate way of
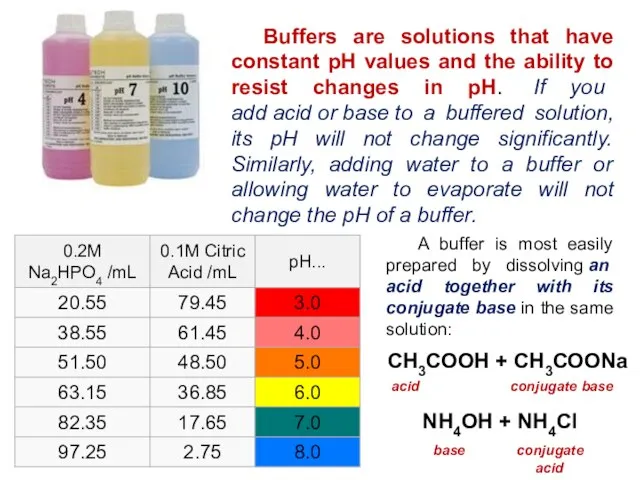
- 21. Buffers are solutions that have constant pH values and the ability to resist changes in pH.
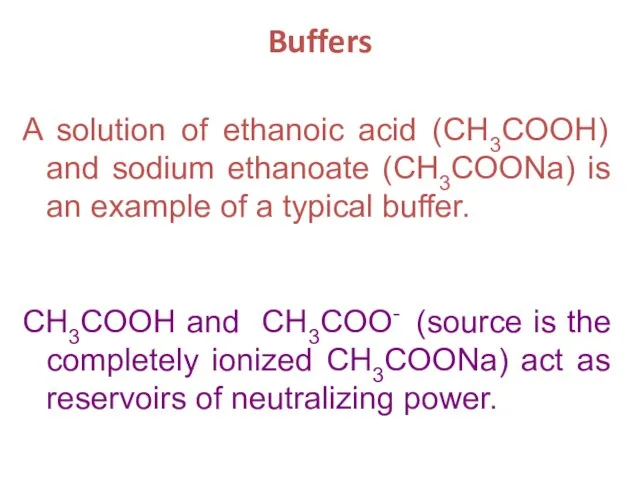
- 22. Buffers A solution of ethanoic acid (CH3COOH) and sodium ethanoate (CH3COONa) is an example of a
- 23. CH3COO-(aq) + H+(aq) CH3COOH (aq) ethanoate ion hydrogen ion ethanoic acid When an acid is added
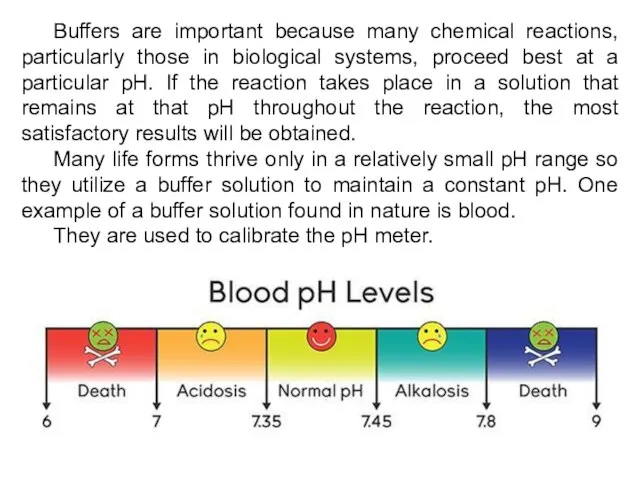
- 24. Buffers are important because many chemical reactions, particularly those in biological systems, proceed best at a
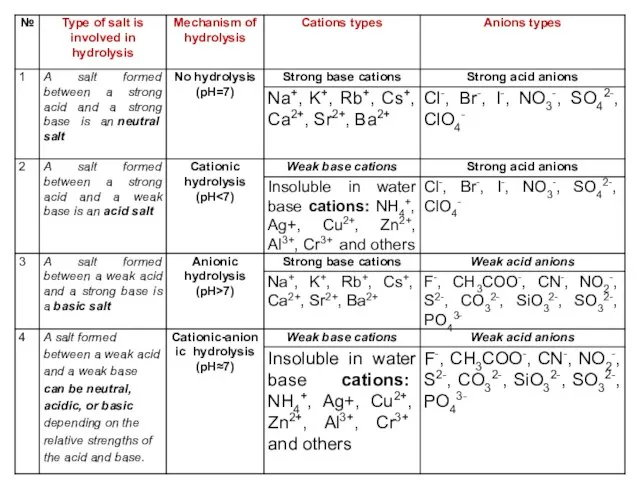
- 26. HYDROLISIS The reaction of salt takes place in the solution. In reality, and looking at a
- 27. A salt is formed between the reaction of an acid and a base. Usually, a neutral
- 28. 3) If the salt is formed from a strong base and weak acid, the salt solution
- 29. If the positive ion (cation) of the salt is from a weak base, it will hydrolyze
- 30. Salt hydrolysis can be described in two chemical equations, the first showing the dissociation of the
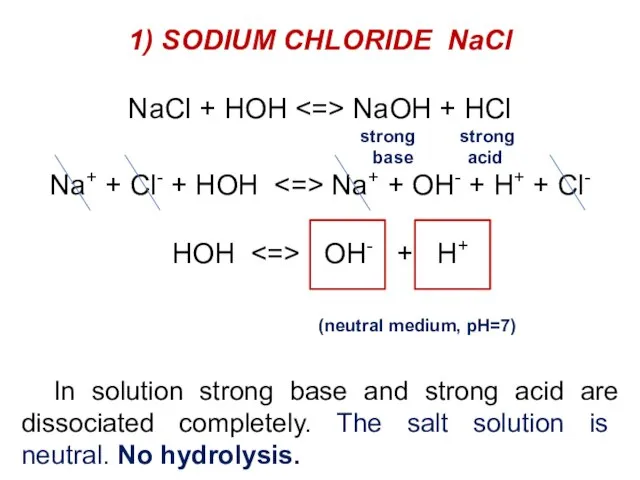
- 31. 1) SODIUM CHLORIDE NaCl NaCl + HOH NaOH + HCl strong strong base acid Na+ +
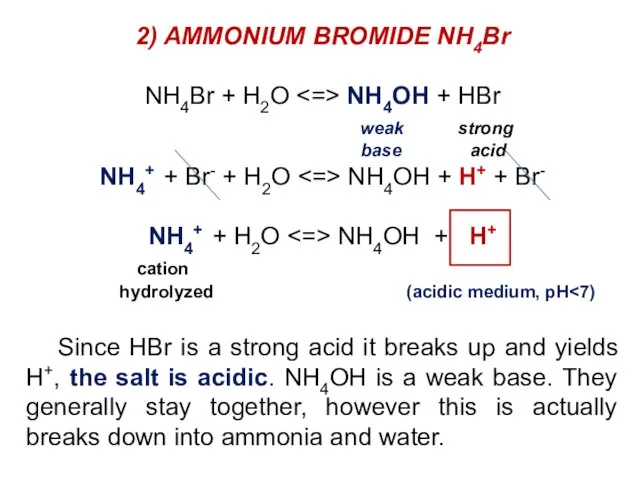
- 32. 2) AMMONIUM BROMIDE NH4Br NH4Br + H2O NH4OH + HBr weak strong base acid NH4+ +
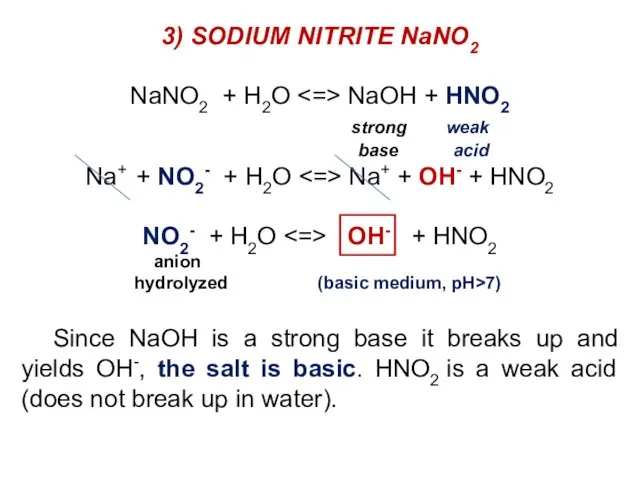
- 33. 3) SODIUM NITRITE NaNO2 NaNO2 + H2O NaOH + HNO2 strong weak base acid Na+ +
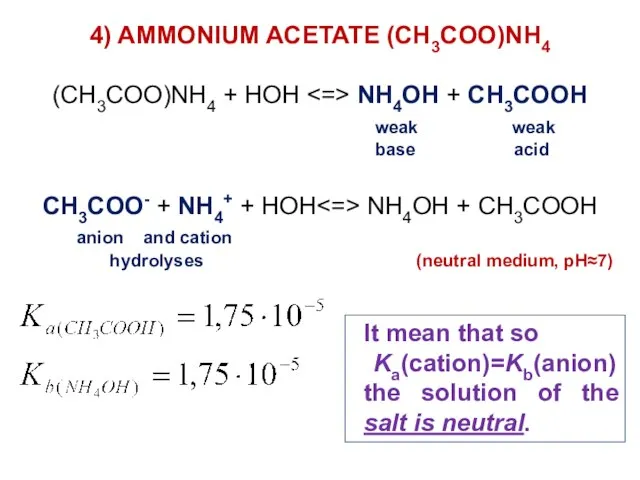
- 34. 4) AMMONIUM ACETATE (CH3COO)NH4 (CH3COO)NH4 + HOH NH4OH + CH3COOH weak weak base acid CH3COO- +
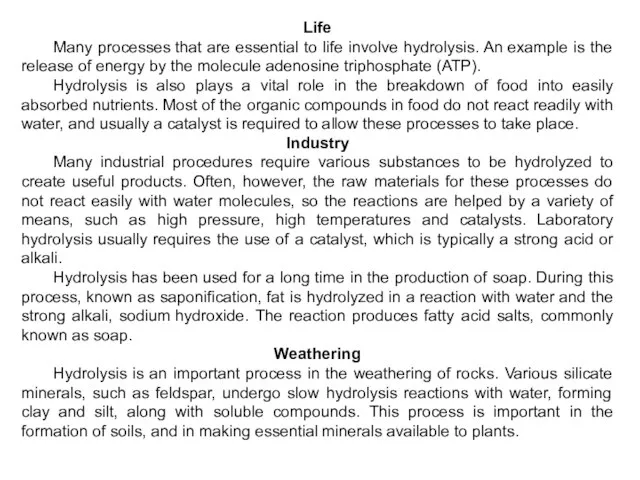
- 36. Life Many processes that are essential to life involve hydrolysis. An example is the release of
- 37. GLOSSARY Acid – A compound that has a proton or protons that can dissociate in water;
- 38. Base – A compound that has the ability to accept a proton or protons from the
- 39. Conjugate acid. The form of a molecule that has a dissociable proton attached to it. Since
- 40. pH. A measure of the acidity of a solution. It is the negative log (base 10)
- 42. Скачать презентацию


![The pH scale is used to express [H+]](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/559017/slide-10.jpg)
![Classifying Solutions A solution in which [H+] is greater than 1](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/559017/slide-11.jpg)












 Открытие кремния
Открытие кремния История мыловарения. Мыло своими руками
История мыловарения. Мыло своими руками Вода – основа жизни на Земле
Вода – основа жизни на Земле Классификация полимеров
Классификация полимеров Генетическая связь неорганических соединений. 8 класс
Генетическая связь неорганических соединений. 8 класс Происхождение нефти
Происхождение нефти Хімія у створенні нових матеріалів та побуті
Хімія у створенні нових матеріалів та побуті Гигиенические нормативы. Химические факторы окружающей среды
Гигиенические нормативы. Химические факторы окружающей среды Кислоты и их свойства
Кислоты и их свойства Отдаленные последствия токсического воздействия. Гигиена труда в с/х при работе с ядохимикатами
Отдаленные последствия токсического воздействия. Гигиена труда в с/х при работе с ядохимикатами Пластмассы
Пластмассы 6 классов ферментов и тривиальные названия некоторых основных групп
6 классов ферментов и тривиальные названия некоторых основных групп Петрография некоторых распространенных метаморфических пород
Петрография некоторых распространенных метаморфических пород Презентация Дисахариды
Презентация Дисахариды Викторина «Своя игра» в рамках декады по биологии, географии и химии
Викторина «Своя игра» в рамках декады по биологии, географии и химии  Периодическая система элементов Д.И. Менделеева
Периодическая система элементов Д.И. Менделеева Криптонит для растений
Криптонит для растений Физико-химия дисперсных систем
Физико-химия дисперсных систем Алюминий и его сплавы
Алюминий и его сплавы Буферные системы. Классификация буферных растворов
Буферные системы. Классификация буферных растворов Хлор
Хлор Спирты и фенолы
Спирты и фенолы Серебро. Химические свойства
Серебро. Химические свойства Классификация органических веществ. Автор: Русакова А.В. учитель химии МОУ «Гимназия № 19» г. Омска.
Классификация органических веществ. Автор: Русакова А.В. учитель химии МОУ «Гимназия № 19» г. Омска.  Аттестационная работа. Образовательная программа внеурочной деятельности «Химия и физика с детства». (1-4 класс)
Аттестационная работа. Образовательная программа внеурочной деятельности «Химия и физика с детства». (1-4 класс) Степень окисления веществ. Задачи
Степень окисления веществ. Задачи Люминесцентный анализ
Люминесцентный анализ Органічна хімія
Органічна хімія