Содержание
- 2. INTRODUCTION The fundamental program of chemical physics consists in understanding chemical phenomena in terms of the
- 3. INTRODUCTION Chemistry is the study of matter and its properties, the changes that matter undergoes, and
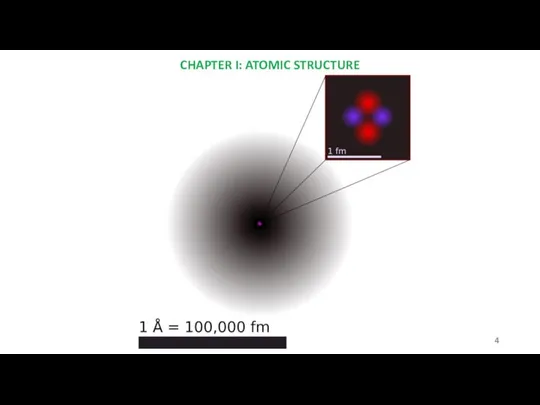
- 4. CHAPTER I: ATOMIC STRUCTURE
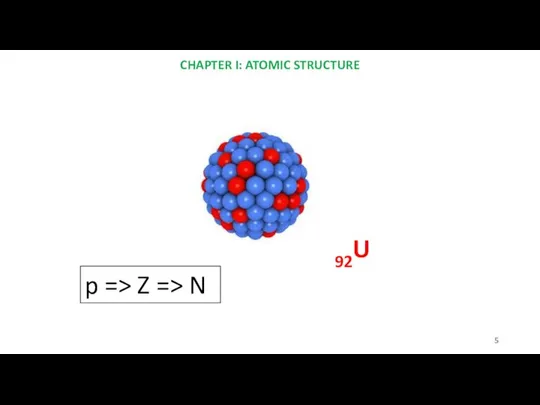
- 5. 92U CHAPTER I: ATOMIC STRUCTURE p => Z => N
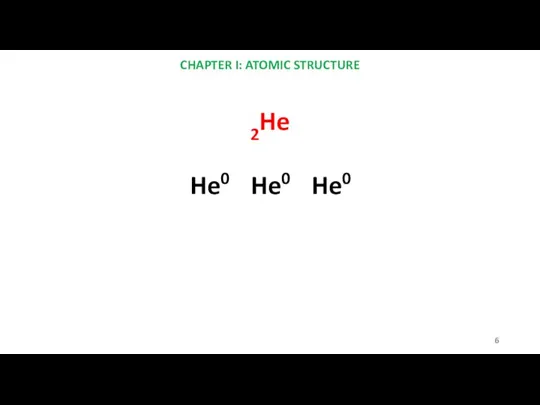
- 6. CHAPTER I: ATOMIC STRUCTURE 2He He0 He0 He0
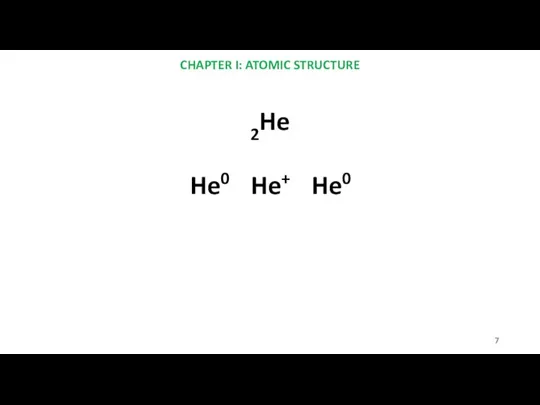
- 7. CHAPTER I: ATOMIC STRUCTURE 2He He0 He+ He0
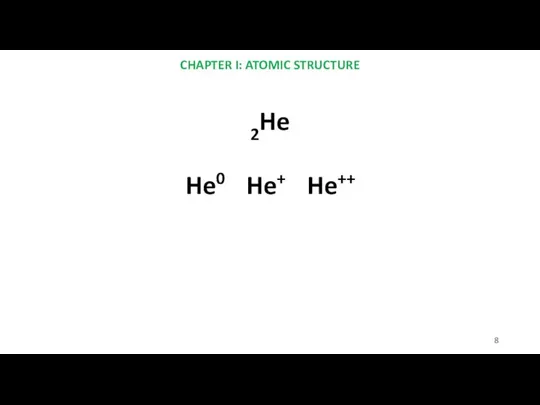
- 8. CHAPTER I: ATOMIC STRUCTURE 2He He0 He+ He++
- 9. CHAPTER I: ATOMIC STRUCTURE 2He He0 He+ He++ Chemical Element: Species of Equicharged Atomic Nuclei
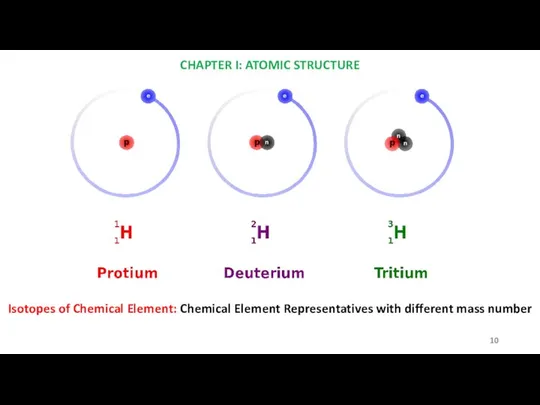
- 10. CHAPTER I: ATOMIC STRUCTURE Isotopes of Chemical Element: Chemical Element Representatives with different mass number
- 11. CHAPTER I: ATOMIC STRUCTURE
- 12. CHAPTER I: ATOMIC STRUCTURE Isotopic Abundance 35·0.7576 + 37·0.2424 = 35.453 [a.m.u] Pie Chart
- 13. CHAPTER I: ATOMIC STRUCTURE
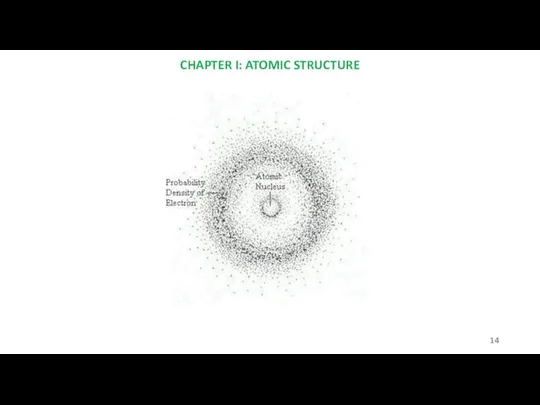
- 14. CHAPTER I: ATOMIC STRUCTURE
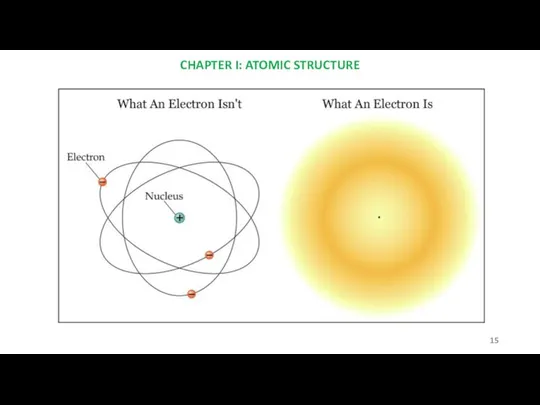
- 15. CHAPTER I: ATOMIC STRUCTURE
- 17. Скачать презентацию



![CHAPTER I: ATOMIC STRUCTURE Isotopic Abundance 35·0.7576 + 37·0.2424 = 35.453 [a.m.u] Pie Chart](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/1395082/slide-11.jpg)
 Амінокислоти
Амінокислоти Углерод. Металлы. 9 класс
Углерод. Металлы. 9 класс Нефть. Состав и свойства
Нефть. Состав и свойства ЕГЭ Химия. Задание №5
ЕГЭ Химия. Задание №5 Введение в современную биотехнологию . «нет ничего более практичного, чем хорошая теория» кто-то из великих физиков Планк ил
Введение в современную биотехнологию . «нет ничего более практичного, чем хорошая теория» кто-то из великих физиков Планк ил Rectification. Difference between the rectification and distillation
Rectification. Difference between the rectification and distillation Автомобильные бензины и дизельное топливо
Автомобильные бензины и дизельное топливо Цифровые лаборатории «Архимед» в изучении биологии и химии
Цифровые лаборатории «Архимед» в изучении биологии и химии Свойства НЦ
Свойства НЦ Тема. Водородная связь
Тема. Водородная связь  ОСОБЕННОСТИ СТРОЕНИЯ, РЕАКЦИОННОЙ СПОСОБНОСТИ И МЕТОДЫ СИНТЕЗА АРЕНОВ
ОСОБЕННОСТИ СТРОЕНИЯ, РЕАКЦИОННОЙ СПОСОБНОСТИ И МЕТОДЫ СИНТЕЗА АРЕНОВ Органический синтез на основе углеродсодержащего сырья
Органический синтез на основе углеродсодержащего сырья ОСНОВАНИЕ
ОСНОВАНИЕ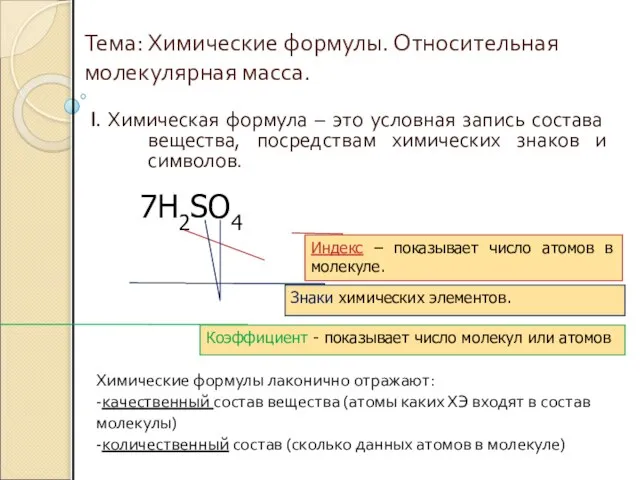 Химические формулы. Относительная молекулярная масса
Химические формулы. Относительная молекулярная масса Алмаз
Алмаз Иондық емес флокулянттар
Иондық емес флокулянттар Оксид азота(2) NO
Оксид азота(2) NO Мийна дія мила Степанова Євстаф’єва
Мийна дія мила Степанова Євстаф’єва  Воздух - источник жизни на земле. (3 класс)
Воздух - источник жизни на земле. (3 класс) Кислотоустойчивость пробиотических культур
Кислотоустойчивость пробиотических культур Электролиз. Применение
Электролиз. Применение Номенклатура органических соединений
Номенклатура органических соединений Аналитическая химия. Качественный анализ
Аналитическая химия. Качественный анализ Азот и его соединения
Азот и его соединения Термическая обработка. Превращения при непрерывном охлаждении аустенита. Операции термической обработки стали. (Лекция 7)
Термическая обработка. Превращения при непрерывном охлаждении аустенита. Операции термической обработки стали. (Лекция 7) Определение подлинности лекарственных веществ
Определение подлинности лекарственных веществ Диаграммы бинарных систем, образующих твердые растворы
Диаграммы бинарных систем, образующих твердые растворы Сабақтың тақырыбы: донорлыакцепторлы байланыс. Комплексті қосылыстар
Сабақтың тақырыбы: донорлыакцепторлы байланыс. Комплексті қосылыстар