Содержание
- 2. Crystalline solids have a very regular atomic structure: that is, the local positions of atoms with
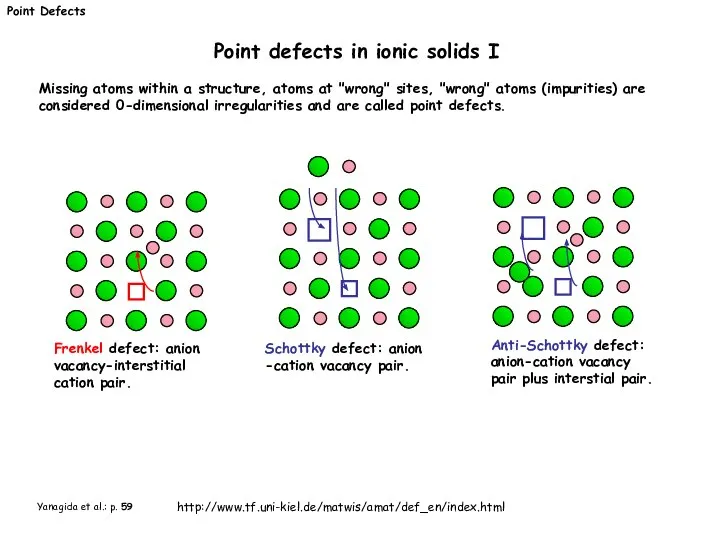
- 3. Point Defects Point defects in ionic solids I Frenkel defect: anion vacancy-interstitial cation pair. Schottky defect:
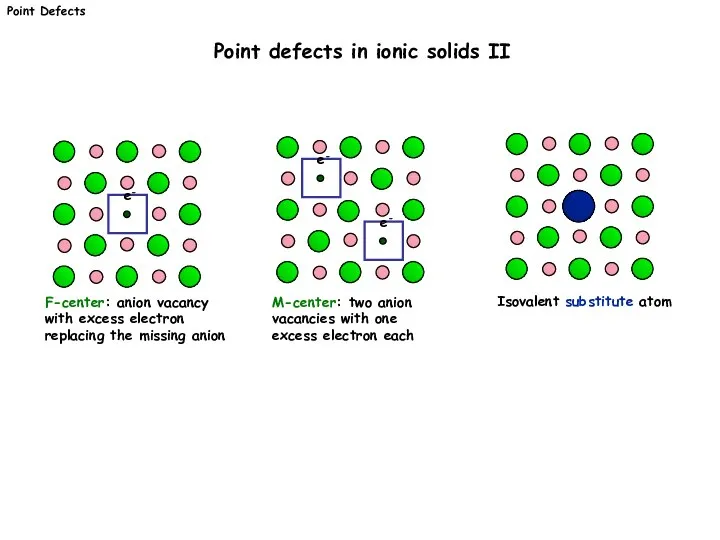
- 4. F-center: anion vacancy with excess electron replacing the missing anion e- M-center: two anion vacancies with
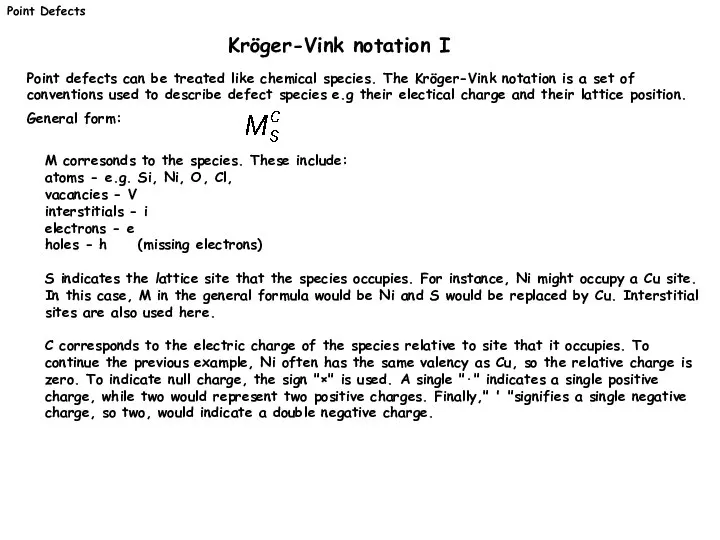
- 5. M corresonds to the species. These include: atoms - e.g. Si, Ni, O, Cl, vacancies -
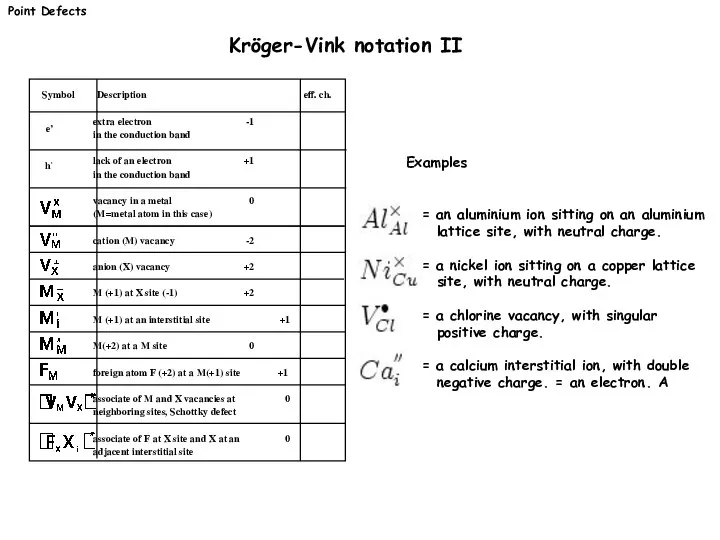
- 6. Point Defects Kröger-Vink notation II = an aluminium ion sitting on an aluminium lattice site, with
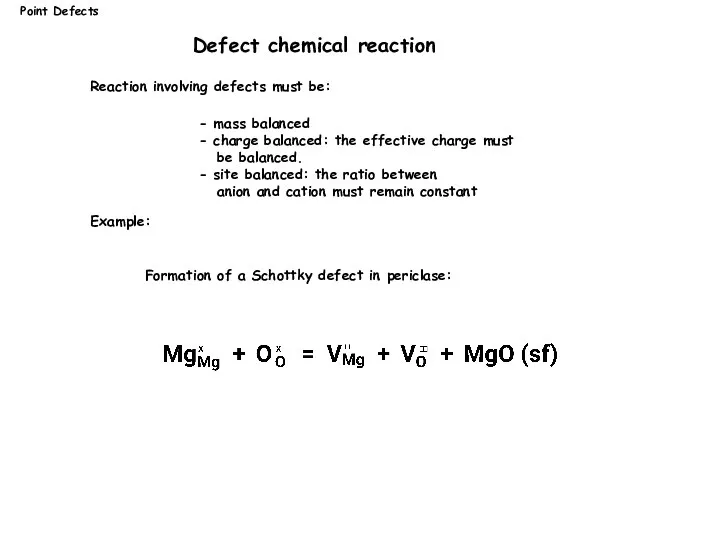
- 7. Reaction involving defects must be: Example: Point Defects Defect chemical reaction Formation of a Schottky defect
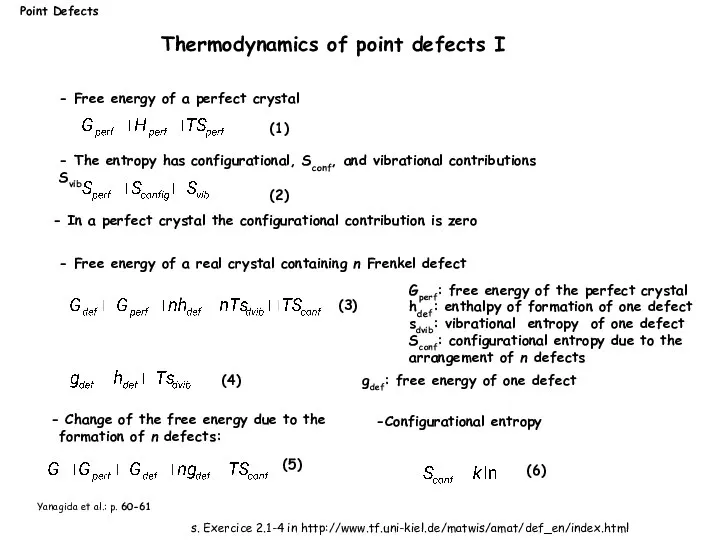
- 8. Gperf: free energy of the perfect crystal hdef: enthalpy of formation of one defect sdvib: vibrational
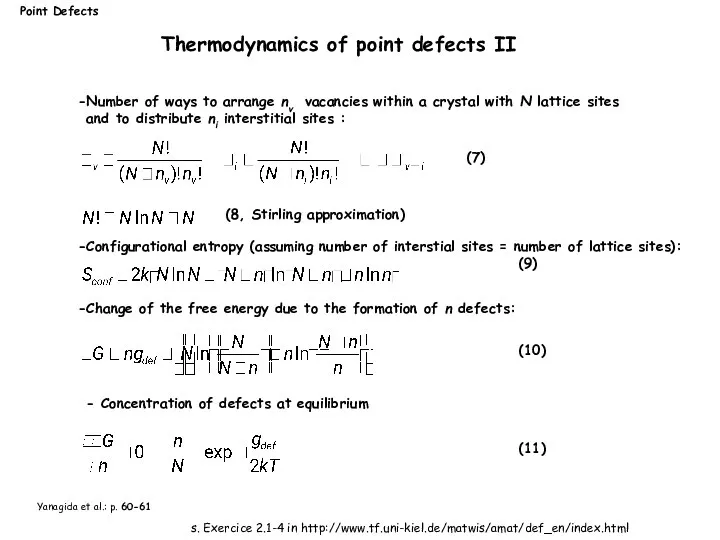
- 9. Yanagida et al.: p. 60-61 Number of ways to arrange nv vacancies within a crystal with
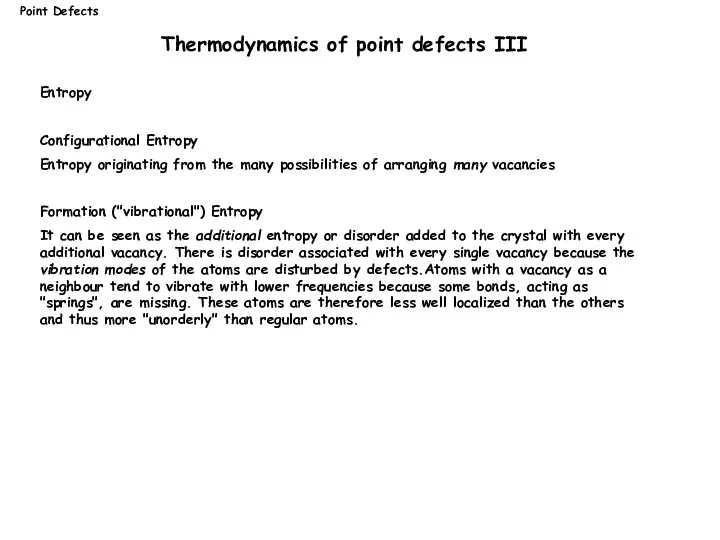
- 10. Entropy Configurational Entropy Entropy originating from the many possibilities of arranging many vacancies Formation ("vibrational") Entropy
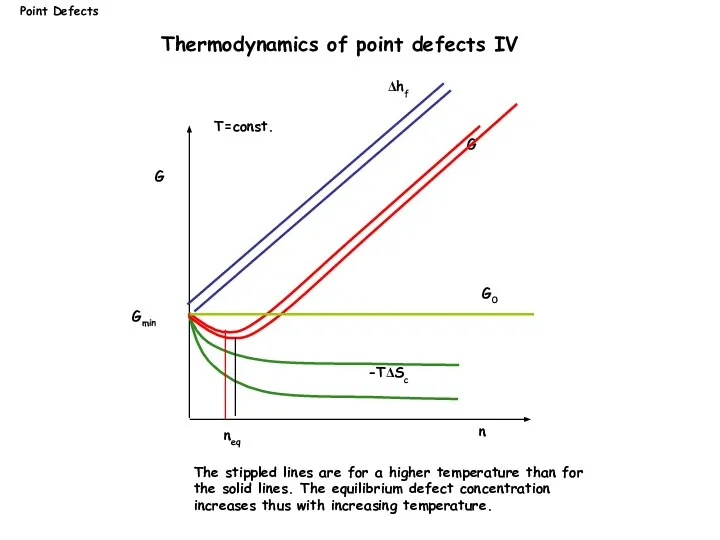
- 11. G n G0 Δhf G neq -TΔSc Gmin T=const. The stippled lines are for a higher
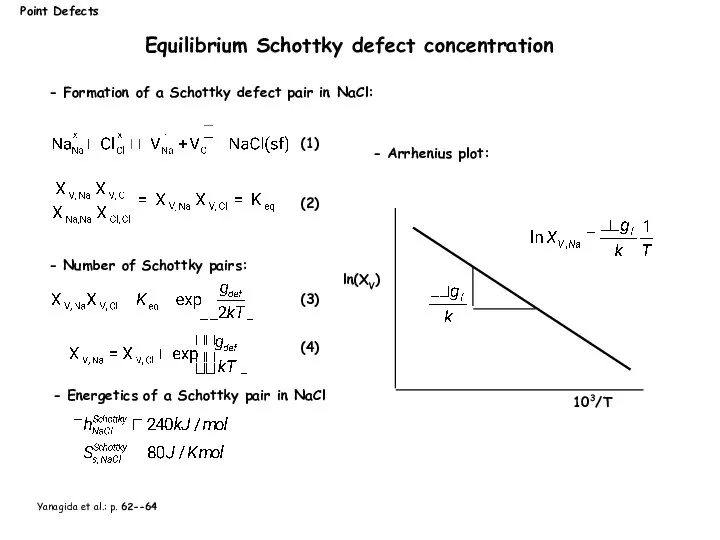
- 12. Point Defects Equilibrium Schottky defect concentration - Number of Schottky pairs: - Formation of a Schottky
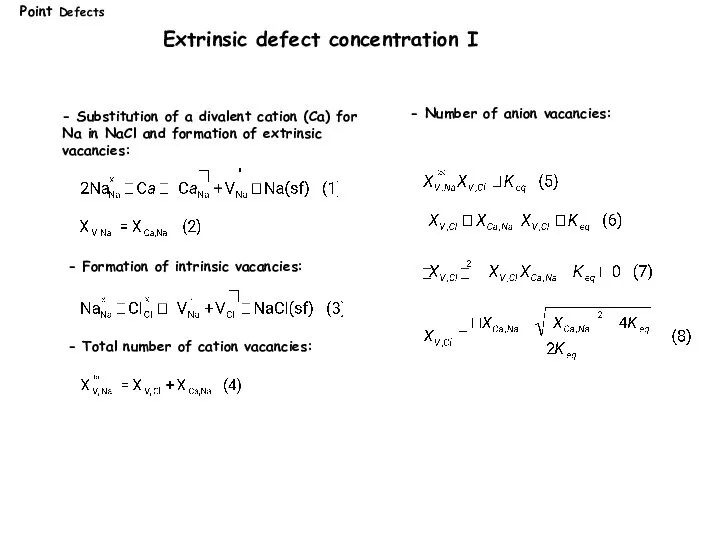
- 13. Extrinsic defect concentration I - Total number of cation vacancies: - Substitution of a divalent cation
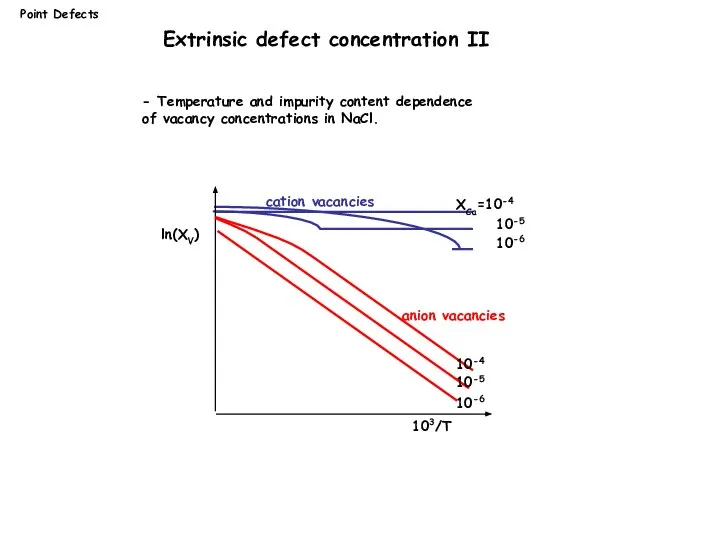
- 14. Point Defects ln(XV) 103/T XCa=10-4 10-5 10-6 10-4 10-5 10-6 cation vacancies anion vacancies - Temperature
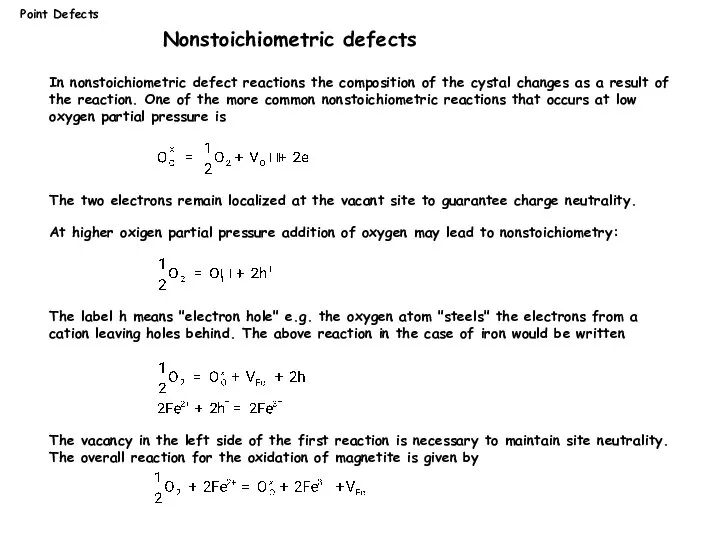
- 15. Point Defects Nonstoichiometric defects In nonstoichiometric defect reactions the composition of the cystal changes as a
- 16. Atomic diffusion is a process whereby the random thermally-activated hopping of atoms in a solid results
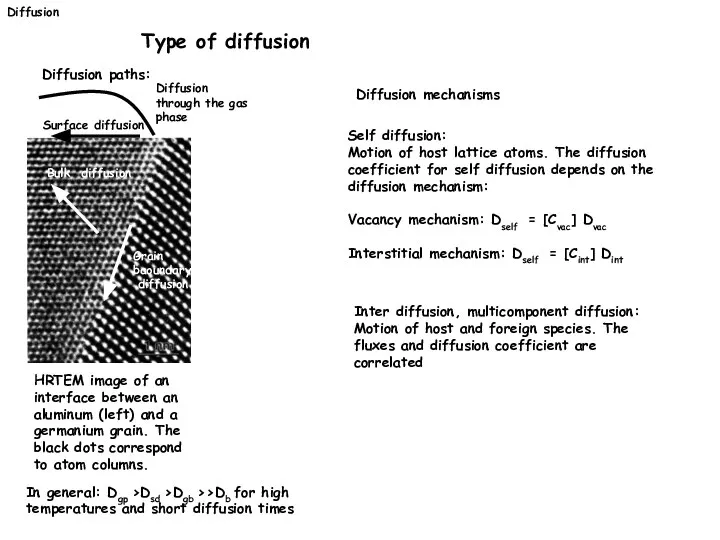
- 17. Diffusion Type of diffusion Diffusion paths: HRTEM image of an interface between an aluminum (left) and
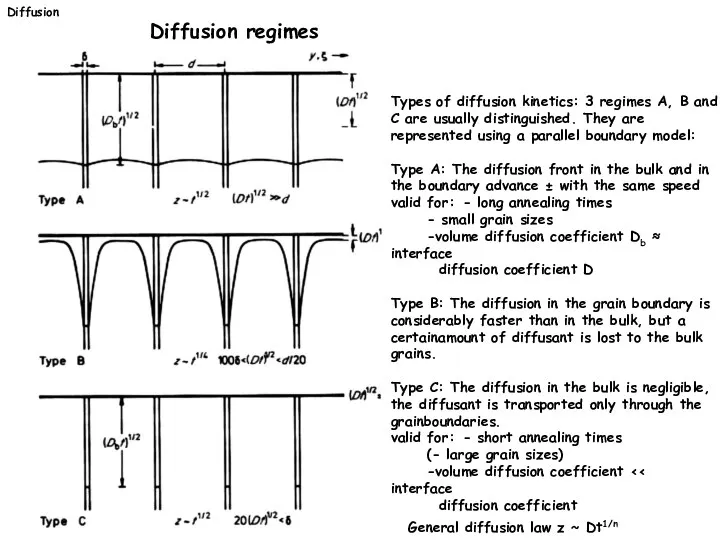
- 18. Types of diffusion kinetics: 3 regimes A, B and C are usually distinguished. They are represented
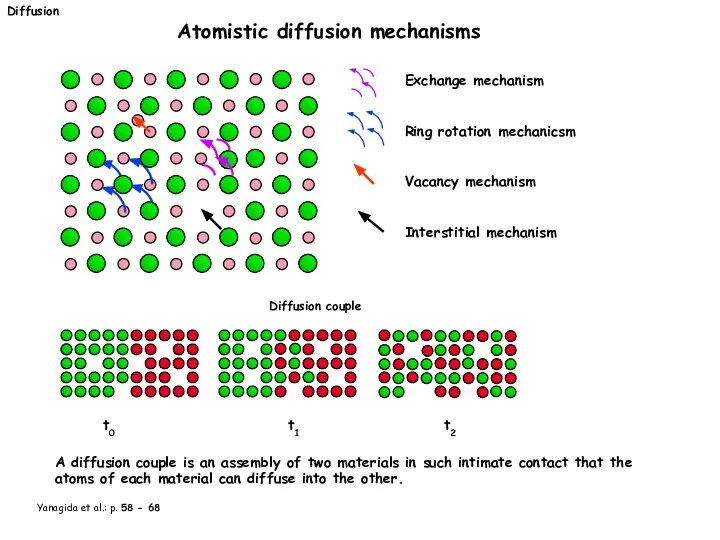
- 19. Diffusion Atomistic diffusion mechanisms Exchange mechanism Ring rotation mechanicsm Vacancy mechanism Interstitial mechanism Diffusion couple t0
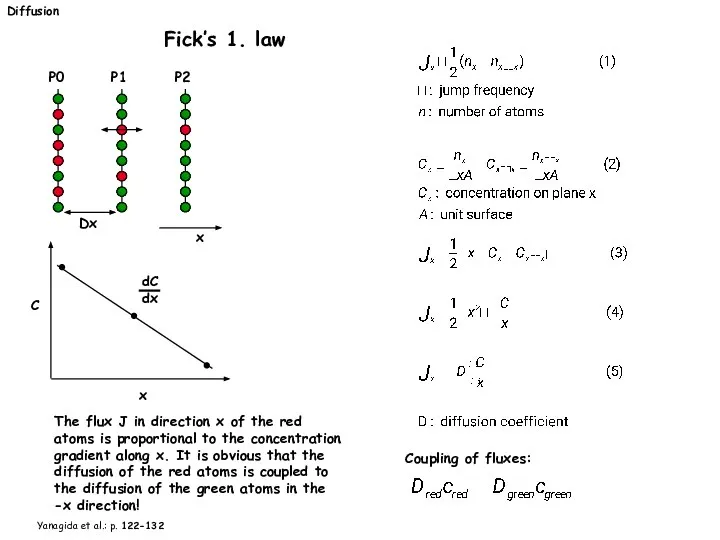
- 20. Diffusion Fick’s 1. law dC dx C x The flux J in direction x of the
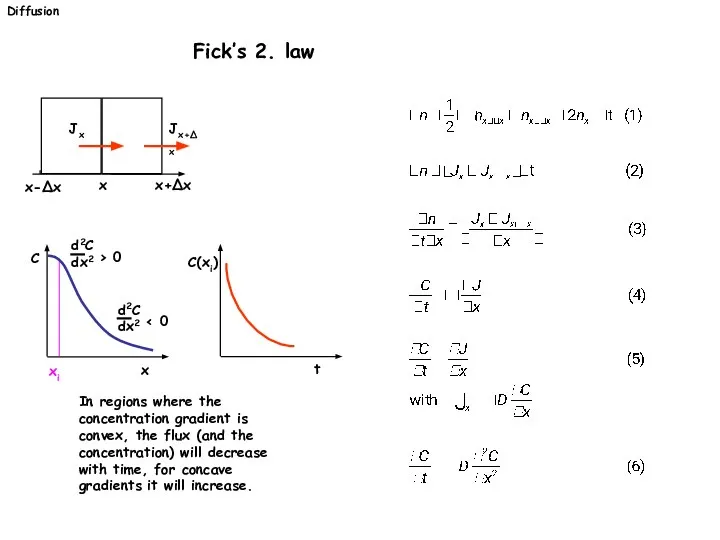
- 21. Diffusion Fick’s 2. law In regions where the concentration gradient is convex, the flux (and the
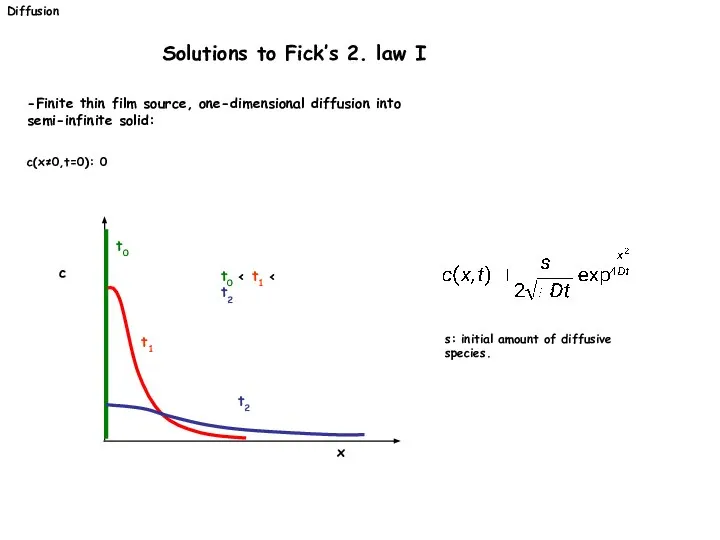
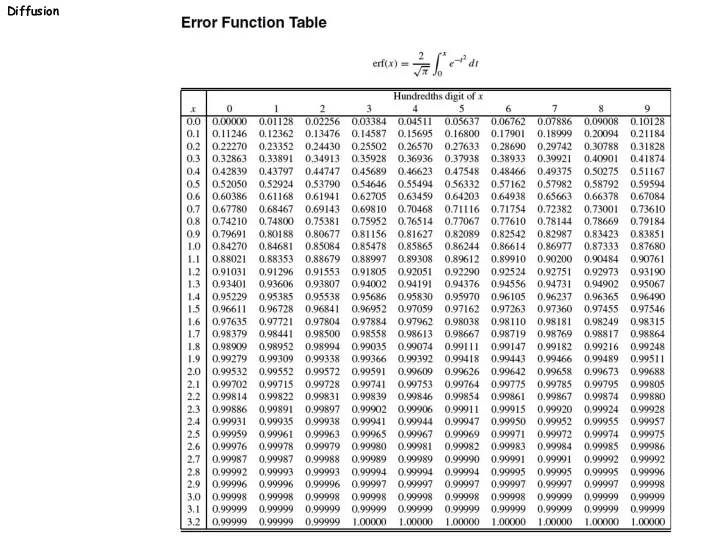
- 22. Diffusion Solutions to Fick’s 2. law I -Finite thin film source, one-dimensional diffusion into semi-infinite solid:
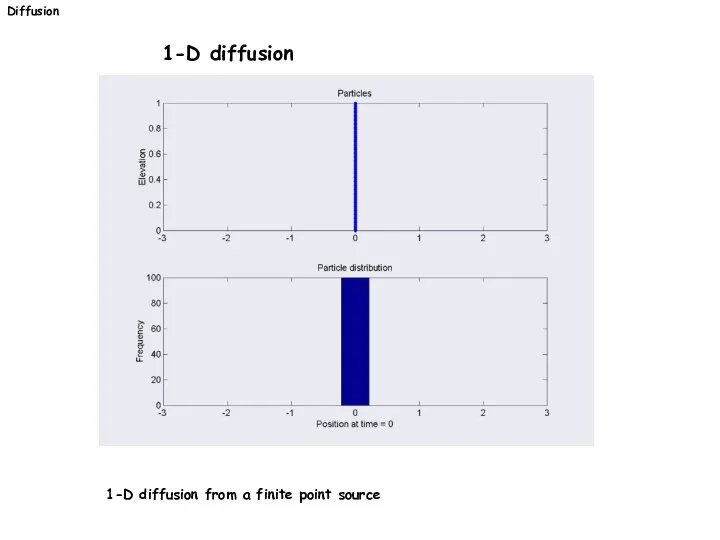
- 23. Diffusion 1-D diffusion 1-D diffusion from a finite point source
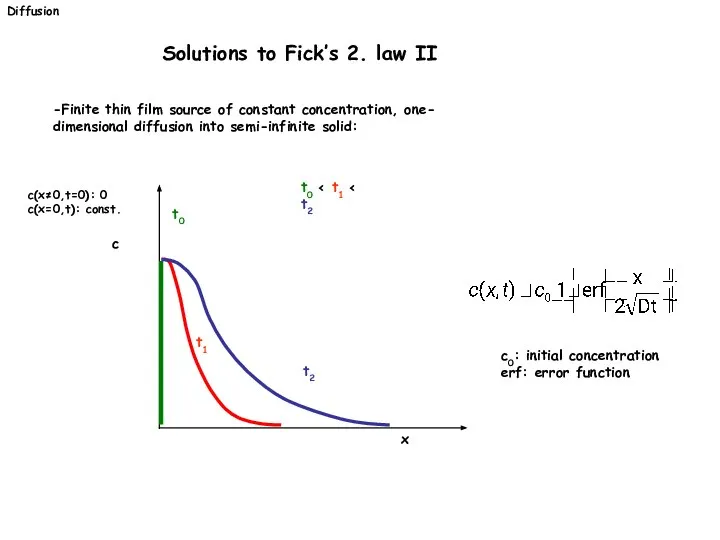
- 24. -Finite thin film source of constant concentration, one- dimensional diffusion into semi-infinite solid: c(x≠0,t=0): 0 c(x=0,t):
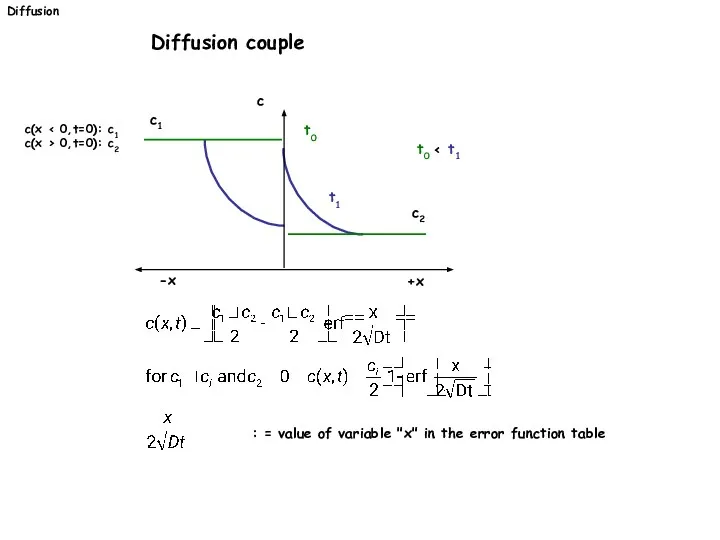
- 25. Diffusion Diffusion couple c(x c(x > 0,t=0): c2 +x -x c t1 t0 t0 c1 c2
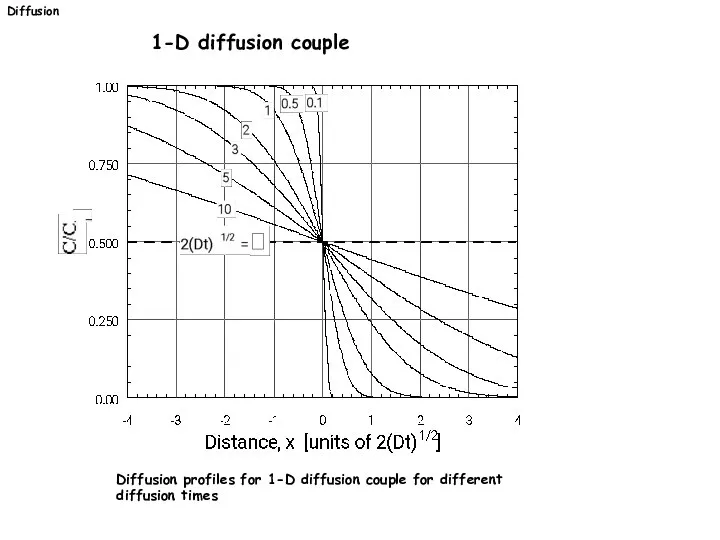
- 26. Diffusion 1-D diffusion couple Diffusion profiles for 1-D diffusion couple for different diffusion times
- 27. Diffusion
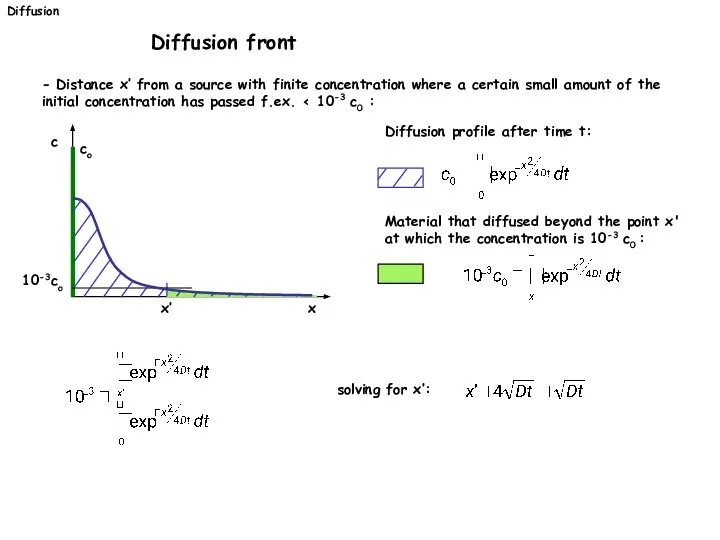
- 28. - Distance x’ from a source with finite concentration where a certain small amount of the
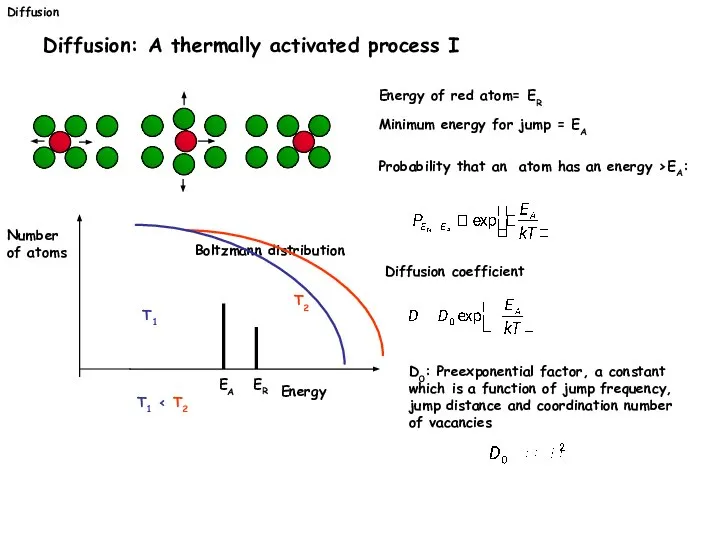
- 29. Diffusion Diffusion: A thermally activated process I Energy of red atom= ER Minimum energy for jump
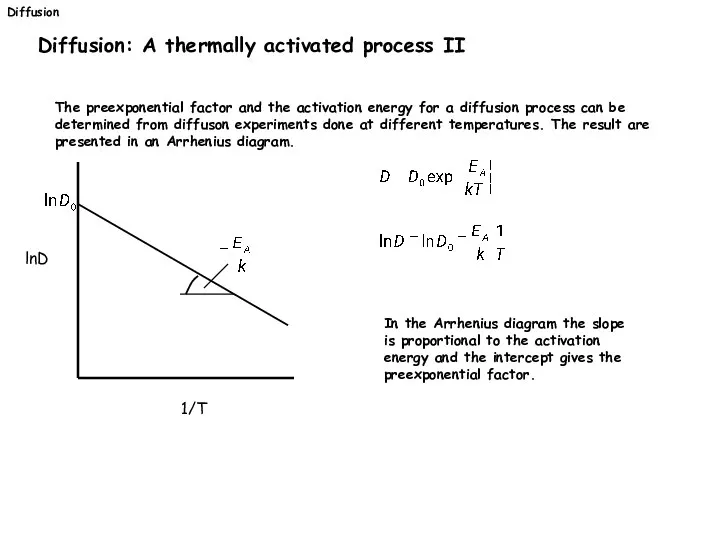
- 30. Diffusion Diffusion: A thermally activated process II The preexponential factor and the activation energy for a
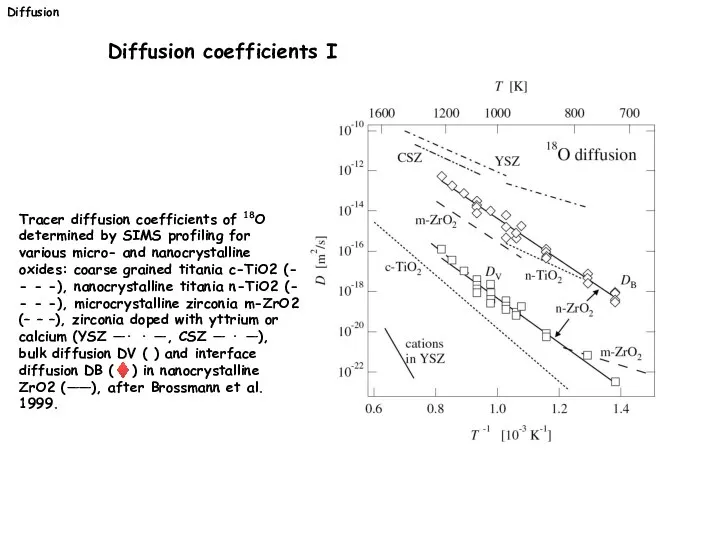
- 31. Tracer diffusion coefficients of 18O determined by SIMS profiling for various micro- and nanocrystalline oxides: coarse
- 33. Скачать презентацию


 Современные положения теории А.М. Бутлерова
Современные положения теории А.М. Бутлерова Химические свойства основных классов неорганических соединений
Химические свойства основных классов неорганических соединений «Уксусная кислота»
«Уксусная кислота»  Исследование минералов в параллельном свете с одним поляризатором
Исследование минералов в параллельном свете с одним поляризатором Биосфера и организм
Биосфера и организм Использование углеводородов в медицине
Использование углеводородов в медицине Хлор
Хлор Коррозия железа
Коррозия железа Расчетная ячейка при МД моделировании. Граничные условия. Элементарная ячейка для атомов аргона
Расчетная ячейка при МД моделировании. Граничные условия. Элементарная ячейка для атомов аргона Model chemistry
Model chemistry Энергетические эффекты реакций
Энергетические эффекты реакций Карбоновые кислоты
Карбоновые кислоты Электронное строение атома
Электронное строение атома EdExcel Unit C2 – Discovering Chemistry
EdExcel Unit C2 – Discovering Chemistry Коррозионные повреждения
Коррозионные повреждения Применение s-, p-, d- элементов в медицине
Применение s-, p-, d- элементов в медицине Насыщенные углеводороды
Насыщенные углеводороды Занимательные опыты
Занимательные опыты Количество вещества. Единица измерения вещества моль
Количество вещества. Единица измерения вещества моль Исследование качества питьевой воды в г. Кашира-8 и способы снижения ее общей жесткости
Исследование качества питьевой воды в г. Кашира-8 и способы снижения ее общей жесткости Гипергенез и почвообразование
Гипергенез и почвообразование Азот. Нахождение в природе
Азот. Нахождение в природе Минералы
Минералы Теоретические основы применения органических реагентов в качественном анализе. (Лекция 8)
Теоретические основы применения органических реагентов в качественном анализе. (Лекция 8) Разложение отходов. 11 класс
Разложение отходов. 11 класс Геохимия рудных месторождений
Геохимия рудных месторождений Аттестационная работа. Способ формирования метапредметных результатов обучения при обучении химии в условиях реализации ФГОС
Аттестационная работа. Способ формирования метапредметных результатов обучения при обучении химии в условиях реализации ФГОС Металлы в природе. Получение и применение металлов
Металлы в природе. Получение и применение металлов