Содержание
- 3. Ретросинтетическая схема
- 4. Синтез фенантренового каркаса (a) guaiacol, Ph3P, DEAD, toluene, 0 °C,87%, >99% ee; (b) i-Bu2AlH, Et2O, hexane,
- 5. Внутримолекулярная реакция Манниха (а) BH3·THF, THF, rt; MeOH, 0 °C; aq NaOH, aq H2O2, 97%; (b)
- 6. Построение бицикло [2,2,2]-скелета KOH, MeOH, 60 °C, 3 h; NaBH4, 0°C, 95%; methyl red, AcCl, MeOH,
- 8. Скачать презентацию
Слайд 2
Слайд 3
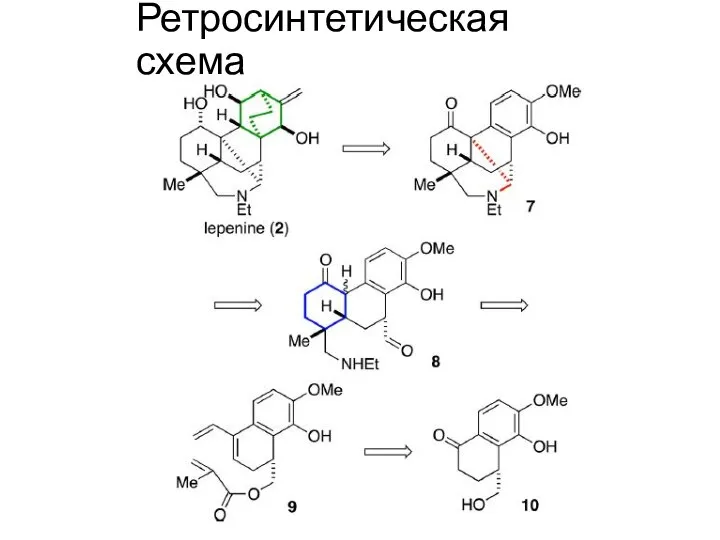
Ретросинтетическая схема
Ретросинтетическая схема
Слайд 4
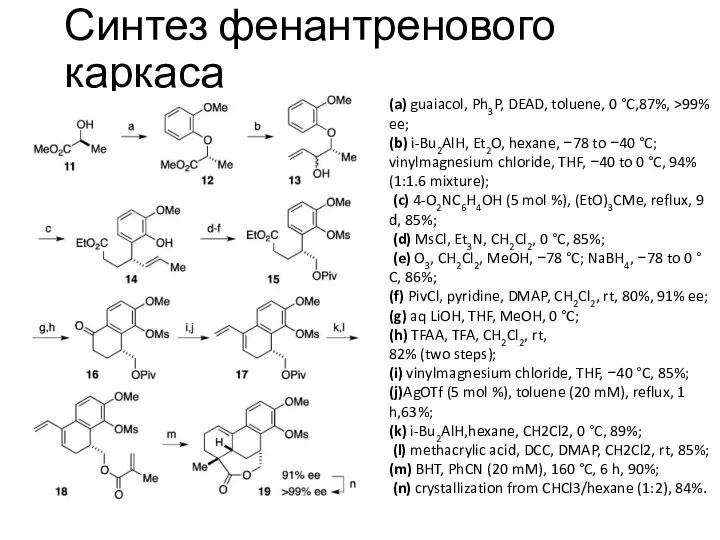
Синтез фенантренового каркаса
(a) guaiacol, Ph3P, DEAD, toluene, 0 °C,87%, >99% ee;
Синтез фенантренового каркаса
(a) guaiacol, Ph3P, DEAD, toluene, 0 °C,87%, >99% ee;
(b) i-Bu2AlH, Et2O, hexane, −78 to −40 °C; vinylmagnesium chloride, THF, −40 to 0 °C, 94% (1:1.6 mixture);
(c) 4-O2NC6H4OH (5 mol %), (EtO)3CMe, reflux, 9 d, 85%;
(d) MsCl, Et3N, CH2Cl2, 0 °C, 85%;
(e) O3, CH2Cl2, MeOH, −78 °C; NaBH4, −78 to 0 °C, 86%;
(f) PivCl, pyridine, DMAP, CH2Cl2, rt, 80%, 91% ee;
(g) aq LiOH, THF, MeOH, 0 °C;
(h) TFAA, TFA, CH2Cl2, rt,
82% (two steps);
(i) vinylmagnesium chloride, THF, −40 °C, 85%;
(j)AgOTf (5 mol %), toluene (20 mM), reflux, 1 h,63%;
(k) i-Bu2AlH,hexane, CH2Cl2, 0 °C, 89%;
(l) methacrylic acid, DCC, DMAP, CH2Cl2, rt, 85%;
(m) BHT, PhCN (20 mM), 160 °C, 6 h, 90%;
(n) crystallization from CHCl3/hexane (1:2), 84%.
Слайд 5
Внутримолекулярная реакция Манниха
(а) BH3·THF, THF, rt; MeOH, 0 °C; aq NaOH,
Внутримолекулярная реакция Манниха
(а) BH3·THF, THF, rt; MeOH, 0 °C; aq NaOH,
aq H2O2, 97%;
(b) i-Bu2AlH, hexane, CH2Cl2, −40 °C, 97%;
(c) EtNH2·HCl, Et3N, AcOH, MeCN, rt; NaBH(OAc)3; aq NaOH, 0°C; AllocCl, 93%;
(d) Dess−Martin periodinane, CH2Cl2, rt, 79%;
(e) Pd(PPh3)4, AcOH, CH2Cl2, reflux, 75%.
(b) i-Bu2AlH, hexane, CH2Cl2, −40 °C, 97%;
(c) EtNH2·HCl, Et3N, AcOH, MeCN, rt; NaBH(OAc)3; aq NaOH, 0°C; AllocCl, 93%;
(d) Dess−Martin periodinane, CH2Cl2, rt, 79%;
(e) Pd(PPh3)4, AcOH, CH2Cl2, reflux, 75%.
Слайд 6
Построение бицикло [2,2,2]-скелета
KOH, MeOH, 60 °C, 3 h; NaBH4, 0°C, 95%;
methyl
Построение бицикло [2,2,2]-скелета
KOH, MeOH, 60 °C, 3 h; NaBH4, 0°C, 95%;
methyl
red, AcCl, MeOH, rt; PhI(OAc)2, 0 °C, 88%;
ethylene (70 bar), CH2Cl2, 70 °C, 5 d, 84%.
ethylene (70 bar), CH2Cl2, 70 °C, 5 d, 84%.
- Предыдущая
Медико-социальная помощьСледующая -
Расчет строительных конструкций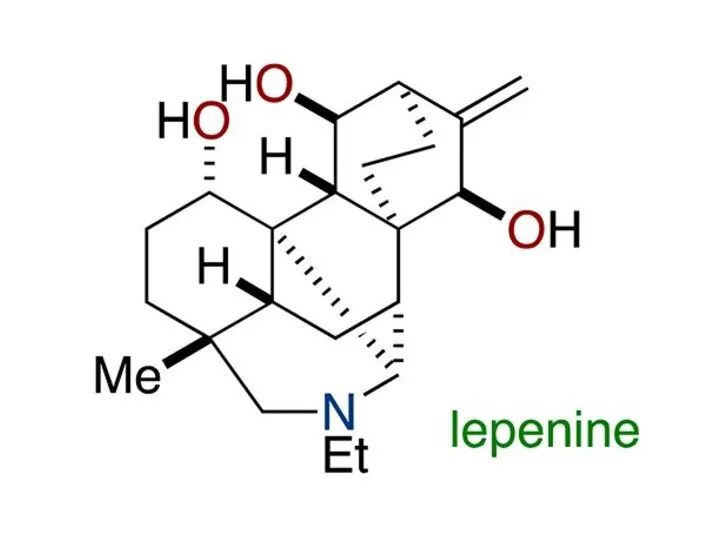

![Построение бицикло [2,2,2]-скелета KOH, MeOH, 60 °C, 3 h; NaBH4, 0°C,](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/1351971/slide-5.jpg)
 Розчинник
Розчинник Кислоты. (8 класс.)
Кислоты. (8 класс.) ОГЭ №2, вопрос 1-16
ОГЭ №2, вопрос 1-16 Общая химия, понятия
Общая химия, понятия Графит. Химические свойства
Графит. Химические свойства Ртуть и здоровье
Ртуть и здоровье Кислоты. Физические свойства кислот
Кислоты. Физические свойства кислот Горение. Подводный факел
Горение. Подводный факел Биологиялық қауіп: экопатогендер, қауіпті биологиялық агенттер, биорегуляторлар. Табиғи токсиндер
Биологиялық қауіп: экопатогендер, қауіпті биологиялық агенттер, биорегуляторлар. Табиғи токсиндер Скорость химических реакций
Скорость химических реакций Генотипическая гетерогенность. Полиморфизм белков. (Лекция 1.2)
Генотипическая гетерогенность. Полиморфизм белков. (Лекция 1.2) Синтетические моющие средства. Механизм моющего действия
Синтетические моющие средства. Механизм моющего действия Химия в повседневной жизни человека
Химия в повседневной жизни человека Тайна мыльного пузыря
Тайна мыльного пузыря Химическая технология органических веществ
Химическая технология органических веществ Урок по химии в 10 классе: «Углерод и кремний – р-элементы IVA-группы» подготовил учитель химии и биологии ГУО СШ №163 г.Минска Ко
Урок по химии в 10 классе: «Углерод и кремний – р-элементы IVA-группы» подготовил учитель химии и биологии ГУО СШ №163 г.Минска Ко Жанн Антуан Ватто
Жанн Антуан Ватто  ИСКУССТВЕННЫЕ ПОЛИМЕРЫ
ИСКУССТВЕННЫЕ ПОЛИМЕРЫ Химическая связь
Химическая связь Химический состав прямогонных бензинов
Химический состав прямогонных бензинов Cвойства цементного камня с введением кольматирующей добавки системы Пенетрон-Адмикс
Cвойства цементного камня с введением кольматирующей добавки системы Пенетрон-Адмикс Регуляция трансляции. Посттрансляционные модификации белков. (Лекция 9)
Регуляция трансляции. Посттрансляционные модификации белков. (Лекция 9) Рентген-флуоресцентті талдау. Микроэлементтер талдауында қолдану
Рентген-флуоресцентті талдау. Микроэлементтер талдауында қолдану Биоорганическая химия
Биоорганическая химия Нуклеиновые кислоты
Нуклеиновые кислоты Исследование Е. Е. Вагнера в области терпенов и камфоры
Исследование Е. Е. Вагнера в области терпенов и камфоры Биополимеры. Липиды
Биополимеры. Липиды Металлы
Металлы