Содержание
- 2. Overview: The Molecules of Life All living things are made up of four classes of large
- 3. Fig. 5-1
- 4. Concept 5.1: Macromolecules are polymers, built from monomers A polymer is a long molecule consisting of
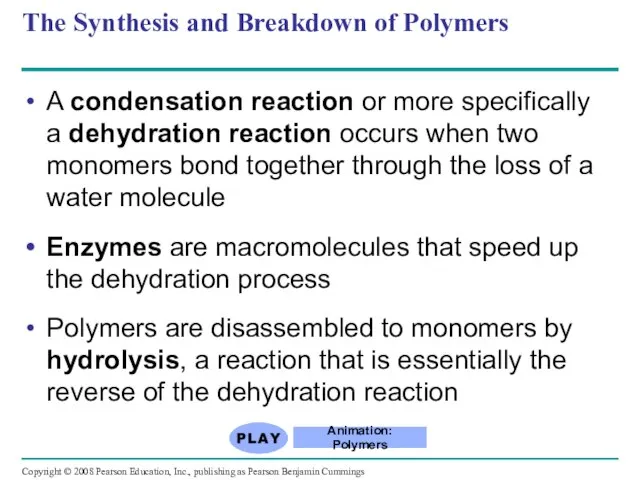
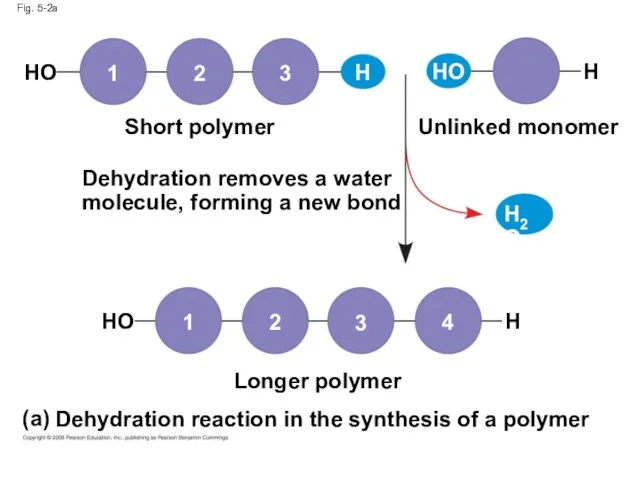
- 5. A condensation reaction or more specifically a dehydration reaction occurs when two monomers bond together through
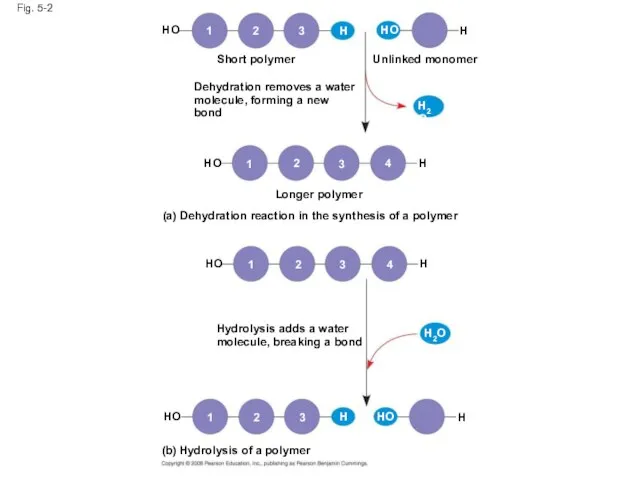
- 6. Fig. 5-2 Short polymer HO 1 2 3 H HO H Unlinked monomer Dehydration removes a
- 7. Fig. 5-2a Dehydration removes a water molecule, forming a new bond Short polymer Unlinked monomer Longer
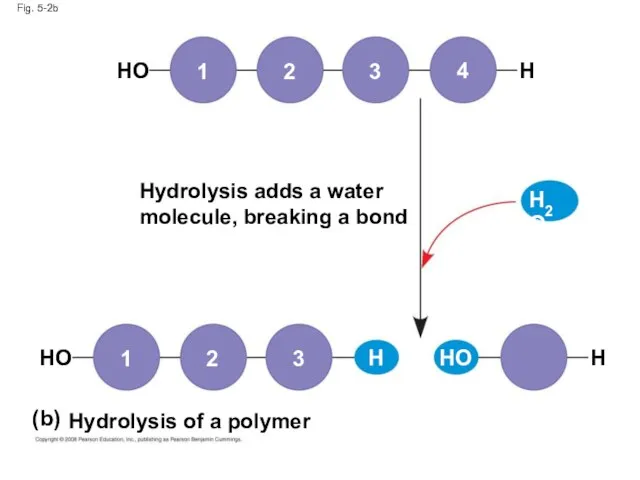
- 8. Fig. 5-2b Hydrolysis adds a water molecule, breaking a bond Hydrolysis of a polymer HO HO
- 9. The Diversity of Polymers Each cell has thousands of different kinds of macromolecules Macromolecules vary among
- 10. Concept 5.2: Carbohydrates serve as fuel and building material Carbohydrates include sugars and the polymers of
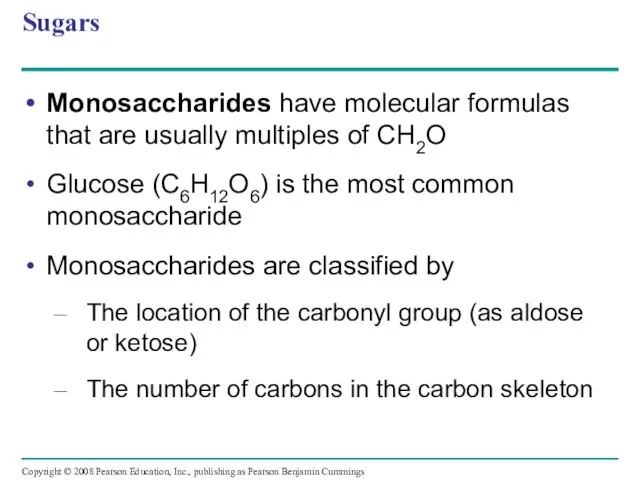
- 11. Sugars Monosaccharides have molecular formulas that are usually multiples of CH2O Glucose (C6H12O6) is the most
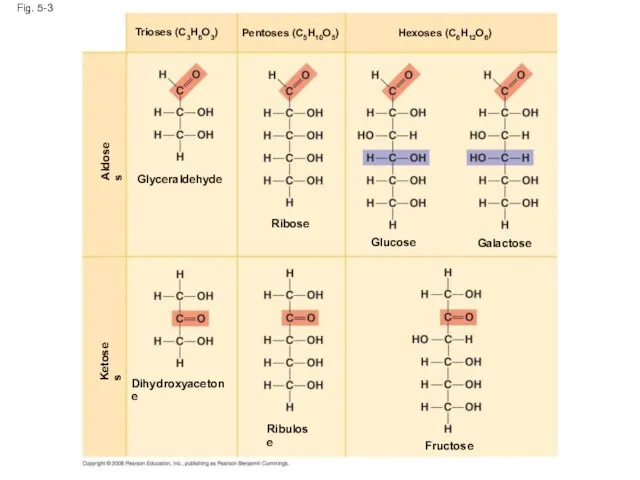
- 12. Fig. 5-3 Dihydroxyacetone Ribulose Ketoses Aldoses Fructose Glyceraldehyde Ribose Glucose Galactose Hexoses (C6H12O6) Pentoses (C5H10O5) Trioses
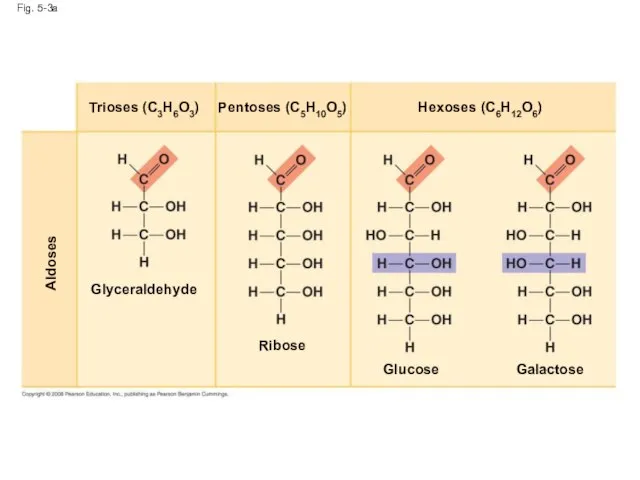
- 13. Fig. 5-3a Aldoses Glyceraldehyde Ribose Glucose Galactose Hexoses (C6H12O6) Pentoses (C5H10O5) Trioses (C3H6O3)
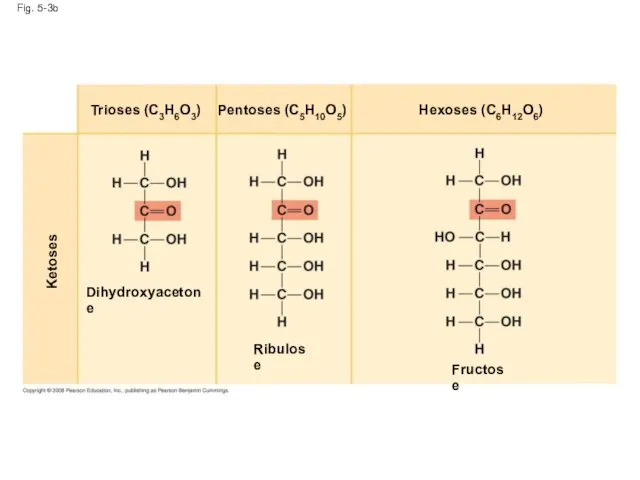
- 14. Fig. 5-3b Ketoses Dihydroxyacetone Ribulose Fructose Hexoses (C6H12O6) Pentoses (C5H10O5) Trioses (C3H6O3)
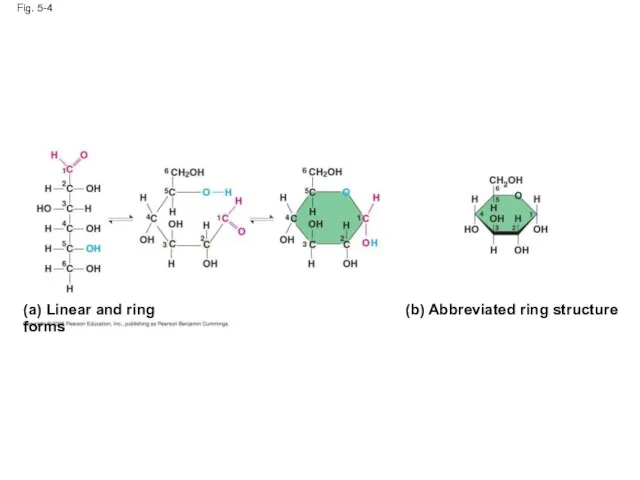
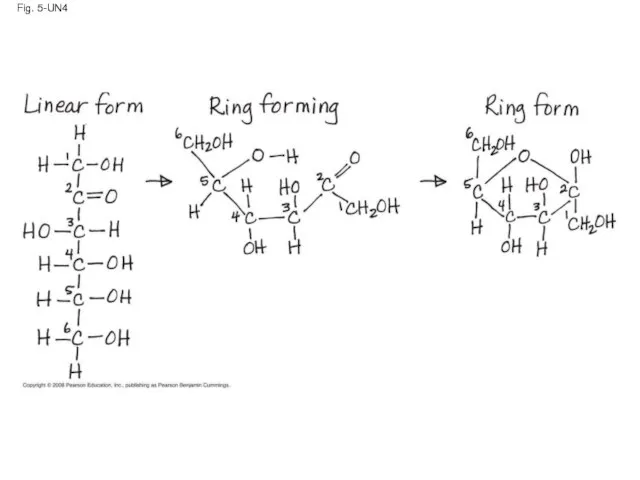
- 15. Though often drawn as linear skeletons, in aqueous solutions many sugars form rings Monosaccharides serve as
- 16. Fig. 5-4 (a) Linear and ring forms (b) Abbreviated ring structure
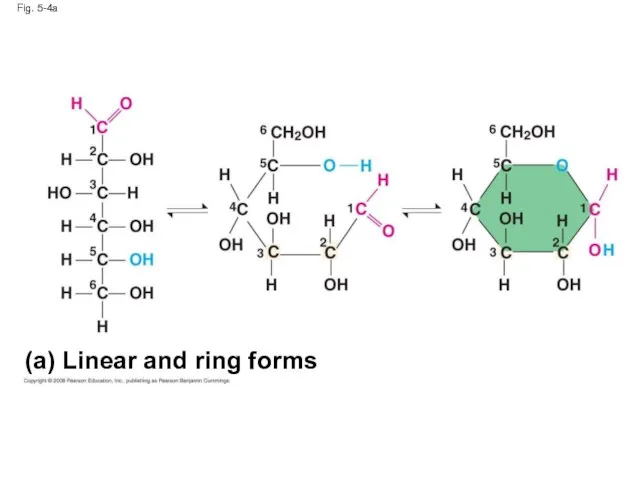
- 17. Fig. 5-4a (a) Linear and ring forms
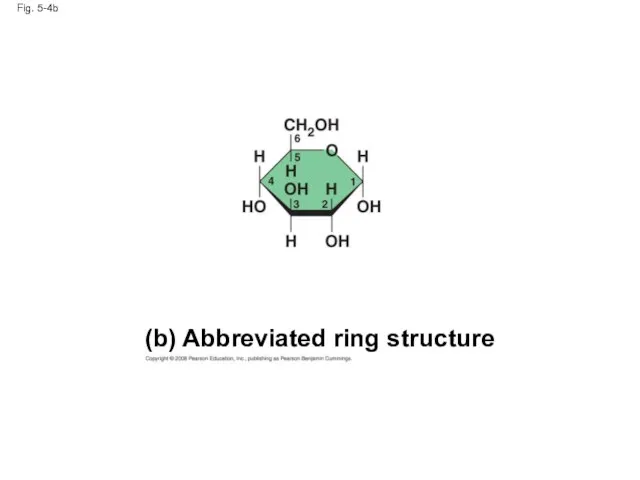
- 18. Fig. 5-4b (b) Abbreviated ring structure
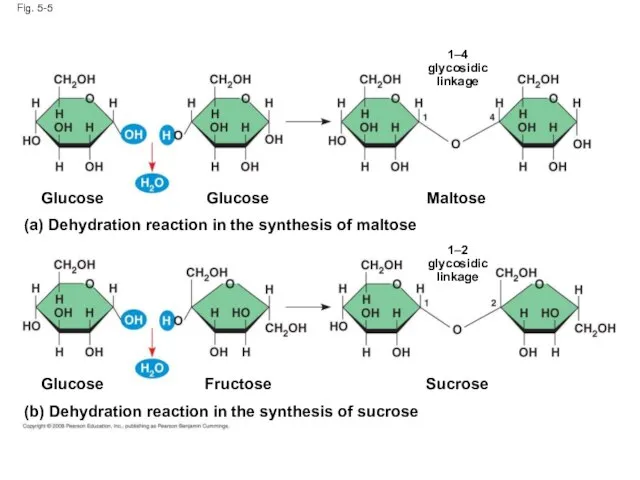
- 19. A disaccharide is formed when a dehydration reaction joins two monosaccharides This covalent bond is called
- 20. Fig. 5-5 (b) Dehydration reaction in the synthesis of sucrose Glucose Fructose Sucrose Maltose Glucose Glucose
- 21. Polysaccharides Polysaccharides, the polymers of sugars, have storage and structural roles The structure and function of
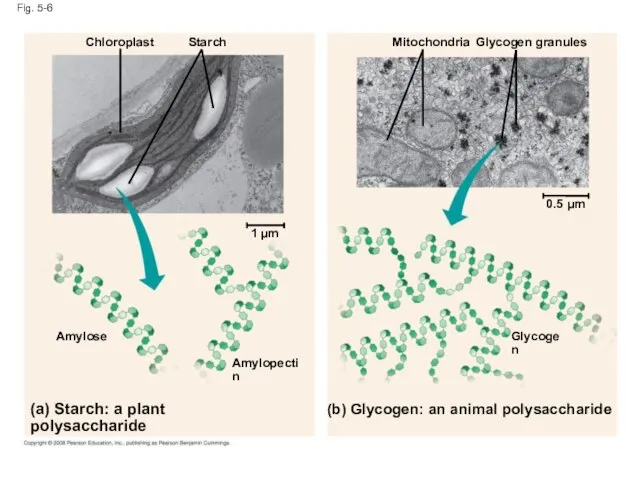
- 22. Storage Polysaccharides Starch, a storage polysaccharide of plants, consists entirely of glucose monomers Plants store surplus
- 23. Fig. 5-6 (b) Glycogen: an animal polysaccharide Starch Glycogen Amylose Chloroplast (a) Starch: a plant polysaccharide
- 24. Glycogen is a storage polysaccharide in animals Humans and other vertebrates store glycogen mainly in liver
- 25. Structural Polysaccharides The polysaccharide cellulose is a major component of the tough wall of plant cells
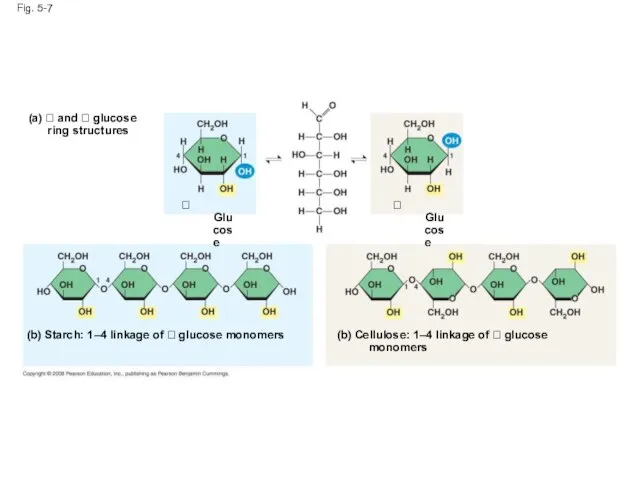
- 26. Fig. 5-7 (a) and glucose ring structures Glucose Glucose (b) Starch: 1–4
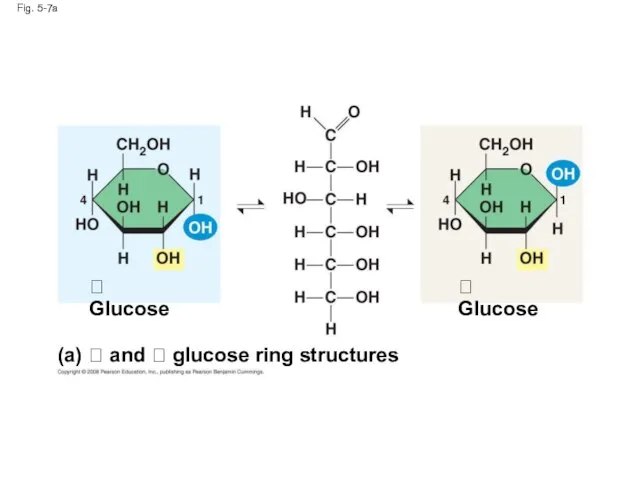
- 27. Fig. 5-7a (a) and glucose ring structures Glucose Glucose
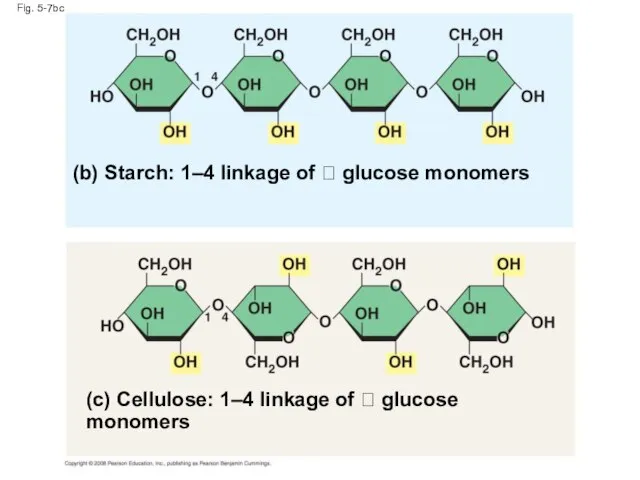
- 28. Fig. 5-7bc (b) Starch: 1–4 linkage of glucose monomers (c) Cellulose: 1–4 linkage of
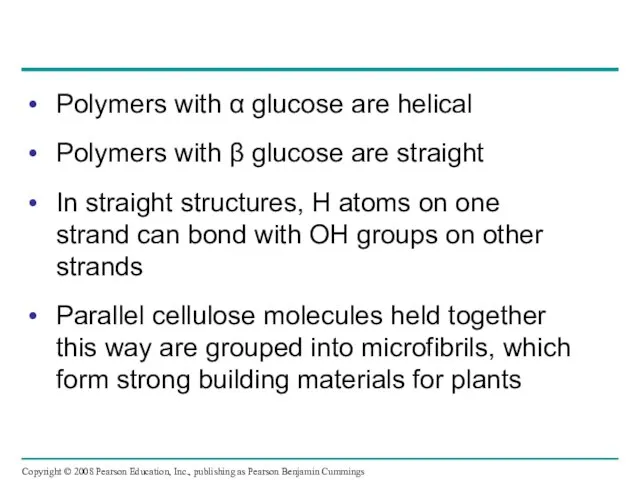
- 29. Polymers with α glucose are helical Polymers with β glucose are straight In straight structures, H
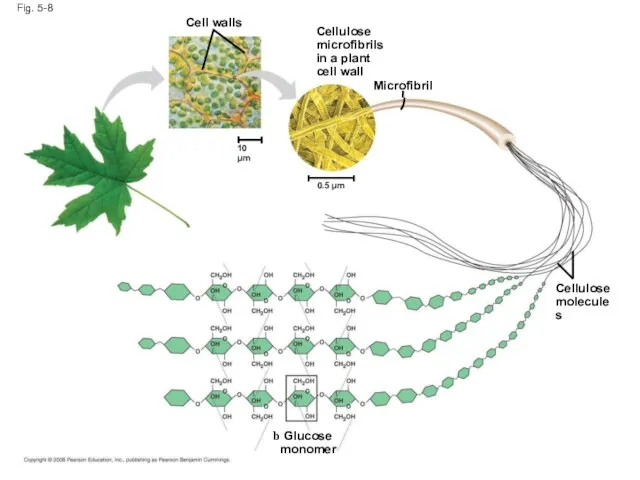
- 30. Fig. 5-8 Glucose monomer Cellulose molecules Microfibril Cellulose microfibrils in a plant cell wall 0.5 µm
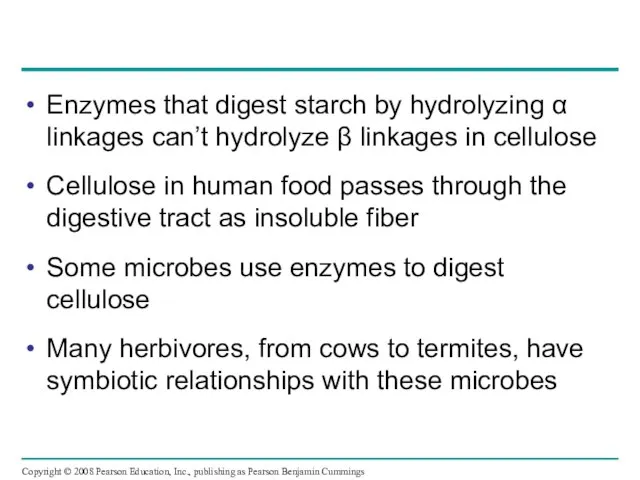
- 31. Enzymes that digest starch by hydrolyzing α linkages can’t hydrolyze β linkages in cellulose Cellulose in
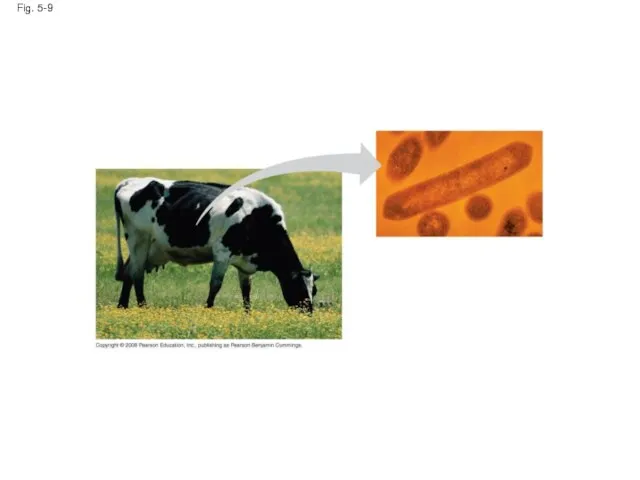
- 32. Fig. 5-9
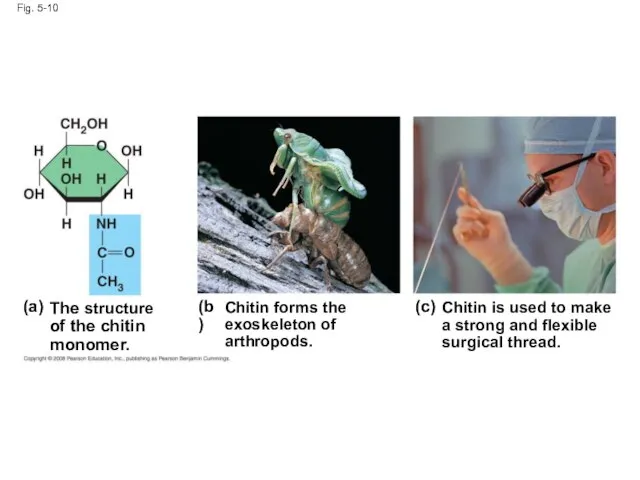
- 33. Chitin, another structural polysaccharide, is found in the exoskeleton of arthropods Chitin also provides structural support
- 34. Fig. 5-10 The structure of the chitin monomer. (a) (b) (c) Chitin forms the exoskeleton of
- 35. Concept 5.3: Lipids are a diverse group of hydrophobic molecules Lipids are the one class of
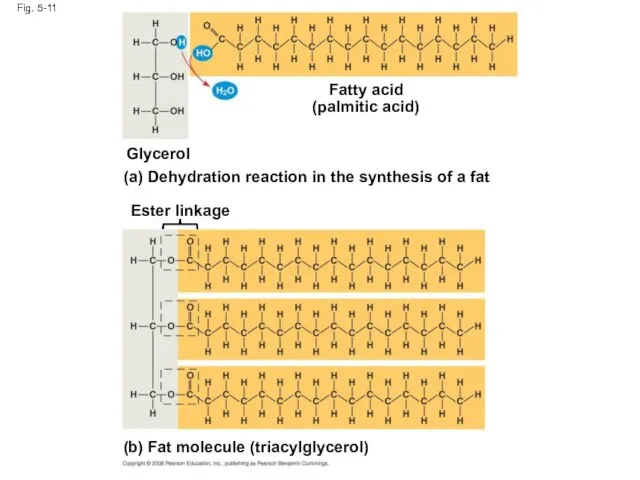
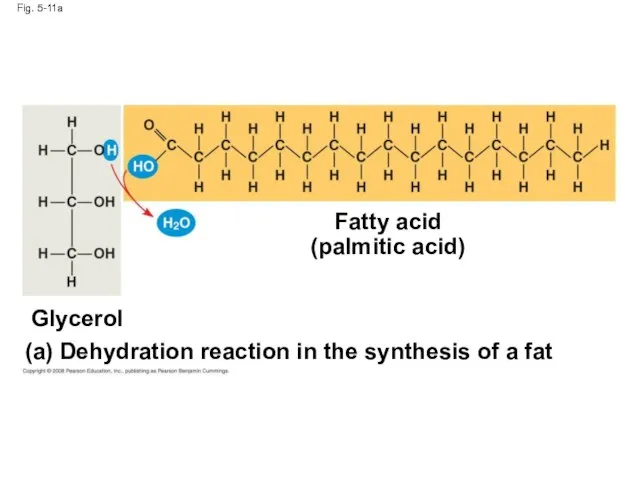
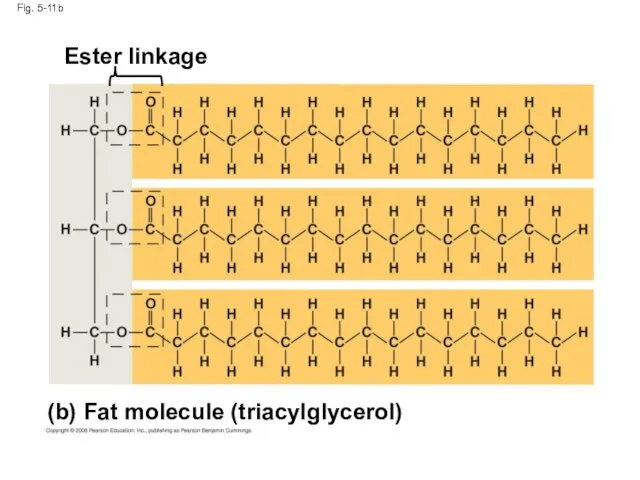
- 36. Fats Fats are constructed from two types of smaller molecules: glycerol and fatty acids Glycerol is
- 37. Fig. 5-11 Fatty acid (palmitic acid) Glycerol (a) Dehydration reaction in the synthesis of a fat
- 38. Fig. 5-11a Fatty acid (palmitic acid) (a) Dehydration reaction in the synthesis of a fat Glycerol
- 39. Fig. 5-11b (b) Fat molecule (triacylglycerol) Ester linkage
- 40. Fats separate from water because water molecules form hydrogen bonds with each other and exclude the
- 41. Fatty acids vary in length (number of carbons) and in the number and locations of double
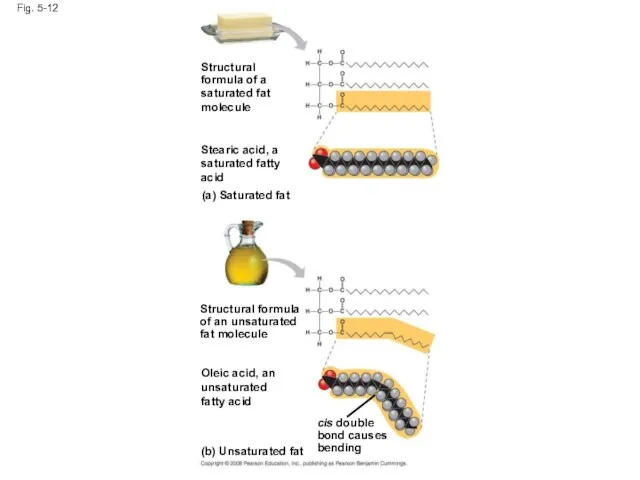
- 42. Fig. 5-12 Structural formula of a saturated fat molecule Stearic acid, a saturated fatty acid (a)
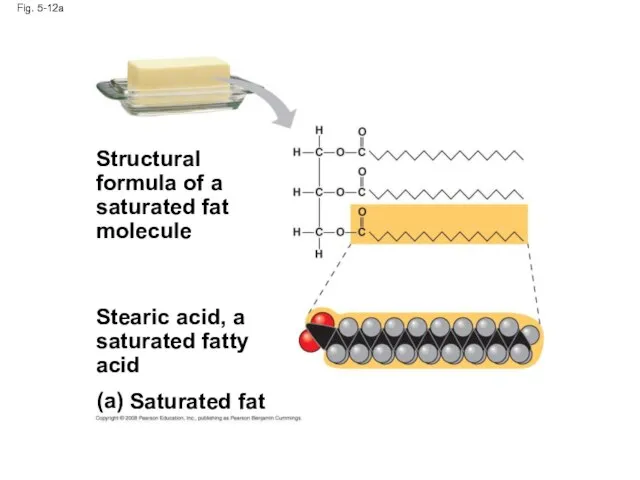
- 43. Fig. 5-12a (a) Saturated fat Structural formula of a saturated fat molecule Stearic acid, a saturated
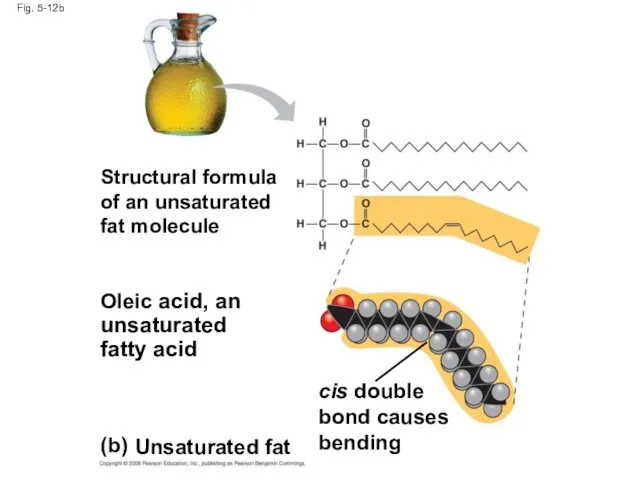
- 44. Fig. 5-12b (b) Unsaturated fat Structural formula of an unsaturated fat molecule Oleic acid, an unsaturated
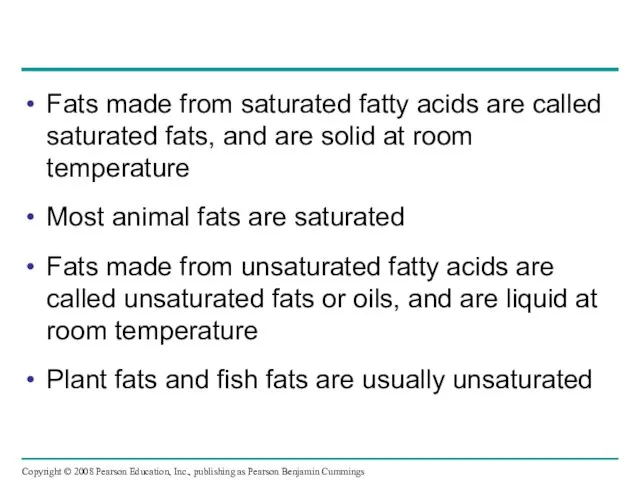
- 45. Fats made from saturated fatty acids are called saturated fats, and are solid at room temperature
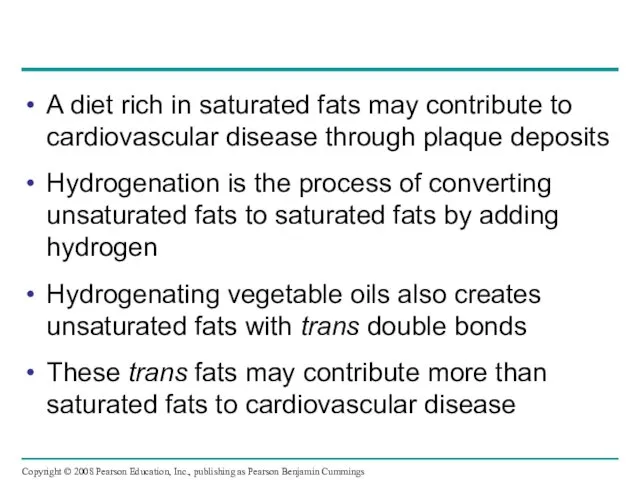
- 46. A diet rich in saturated fats may contribute to cardiovascular disease through plaque deposits Hydrogenation is
- 47. The major function of fats is energy storage Humans and other mammals store their fat in
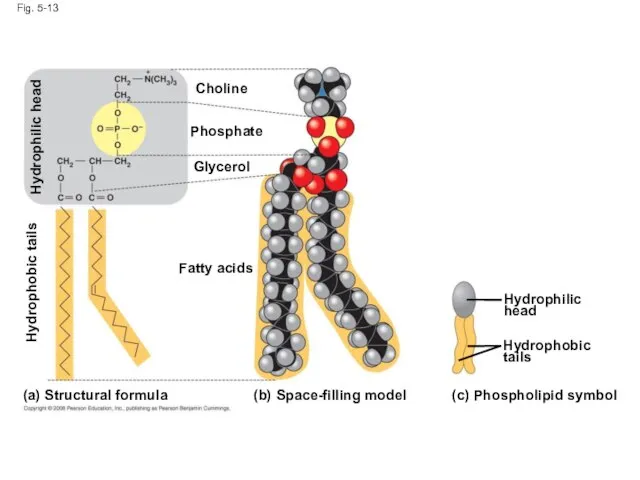
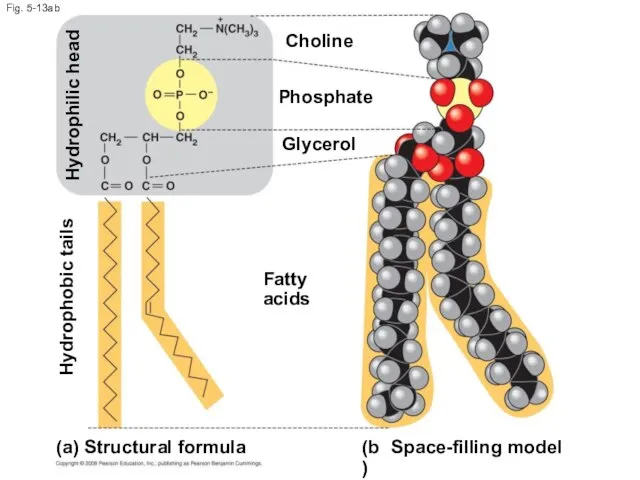
- 48. Phospholipids In a phospholipid, two fatty acids and a phosphate group are attached to glycerol The
- 49. Fig. 5-13 (b) Space-filling model (a) (c) Structural formula Phospholipid symbol Fatty acids Hydrophilic head Hydrophobic
- 50. Fig. 5-13ab (b) Space-filling model (a) Structural formula Fatty acids Choline Phosphate Glycerol Hydrophobic tails Hydrophilic
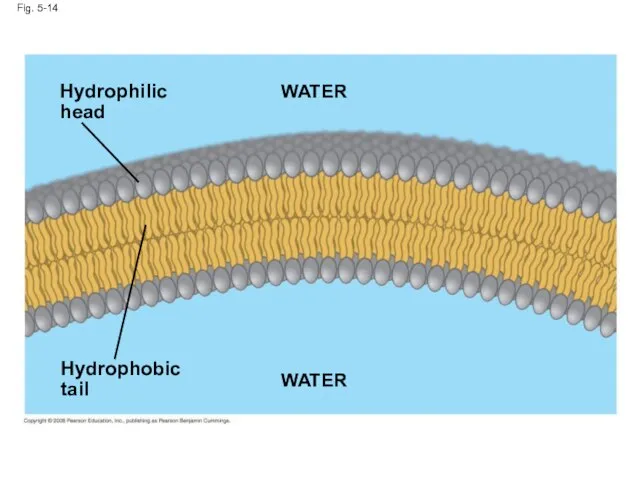
- 51. When phospholipids are added to water, they self-assemble into a bilayer, with the hydrophobic tails pointing
- 52. Fig. 5-14 Hydrophilic head Hydrophobic tail WATER WATER
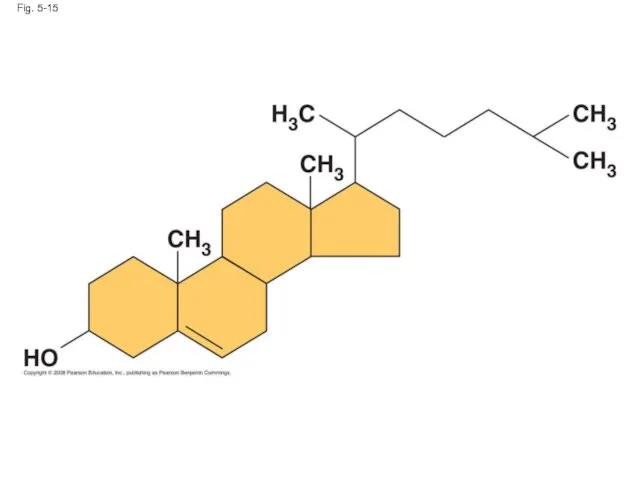
- 53. Steroids Steroids are lipids characterized by a carbon skeleton consisting of four fused rings Cholesterol, an
- 54. Fig. 5-15
- 55. Concept 5.4: Proteins have many structures, resulting in a wide range of functions Proteins account for
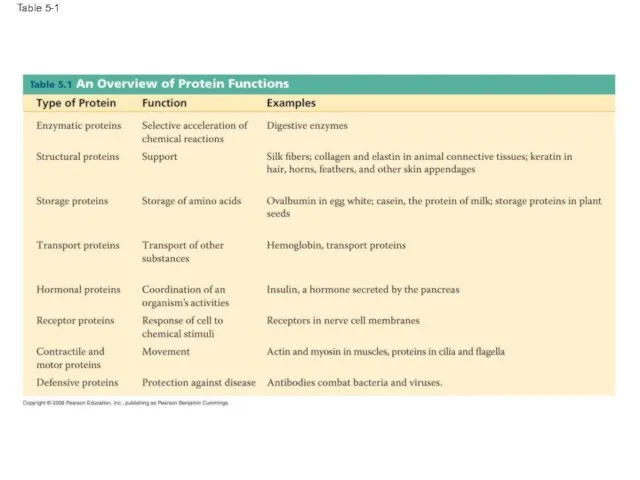
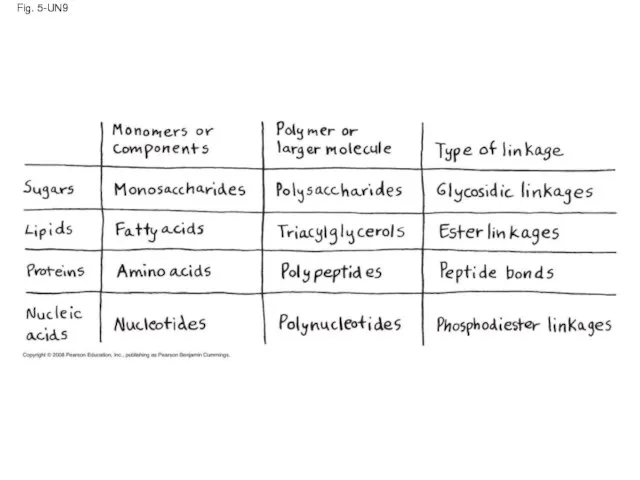
- 56. Table 5-1
- 57. Animation: Structural Proteins Animation: Storage Proteins Animation: Transport Proteins Animation: Receptor Proteins Animation: Contractile Proteins Animation:
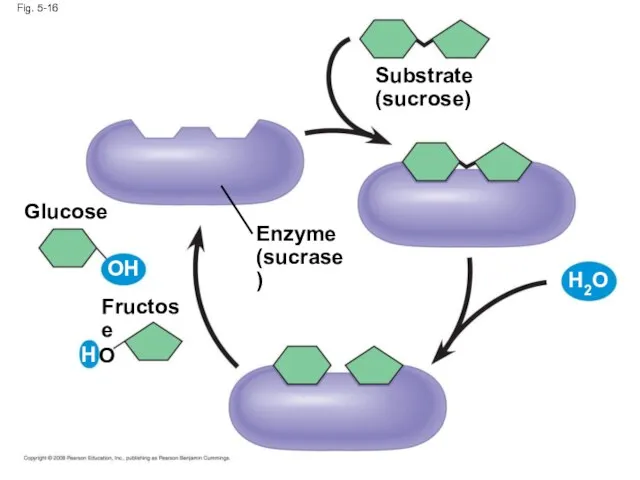
- 58. Enzymes are a type of protein that acts as a catalyst to speed up chemical reactions
- 59. Fig. 5-16 Enzyme (sucrase) Substrate (sucrose) Fructose Glucose OH H O H2O
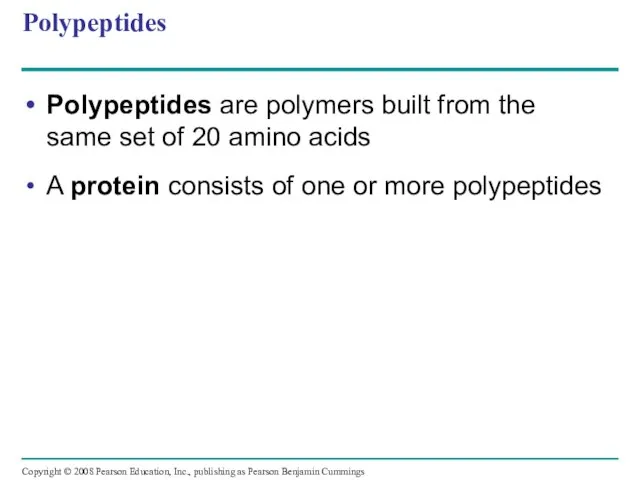
- 60. Polypeptides Polypeptides are polymers built from the same set of 20 amino acids A protein consists
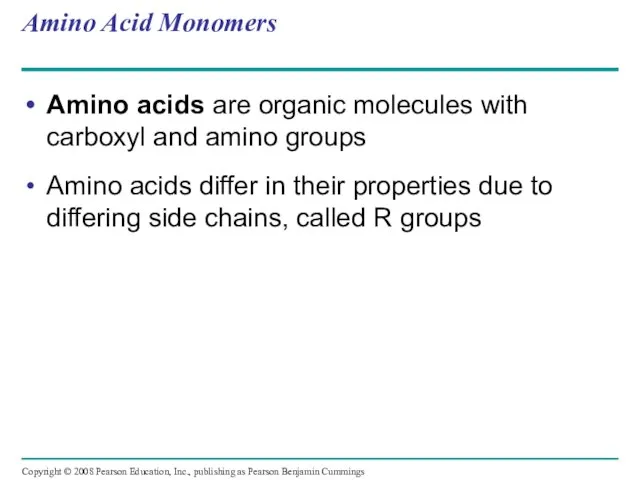
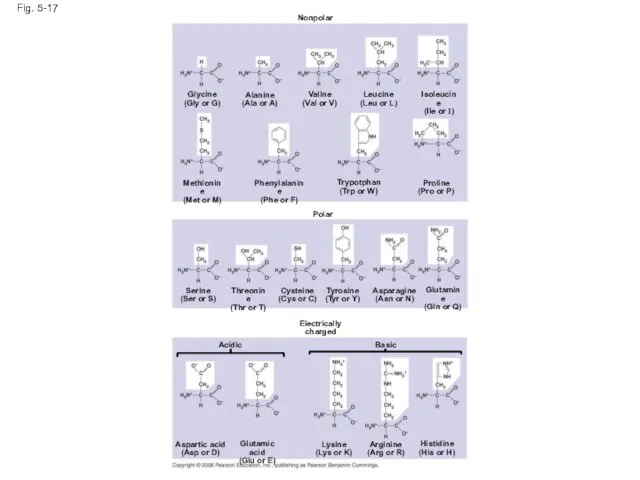
- 61. Amino Acid Monomers Amino acids are organic molecules with carboxyl and amino groups Amino acids differ
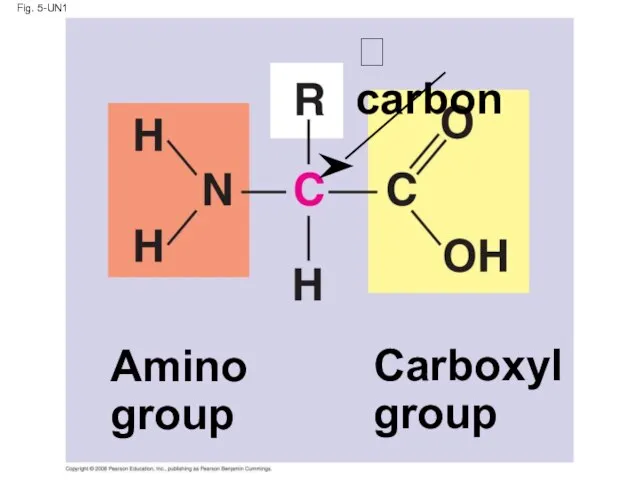
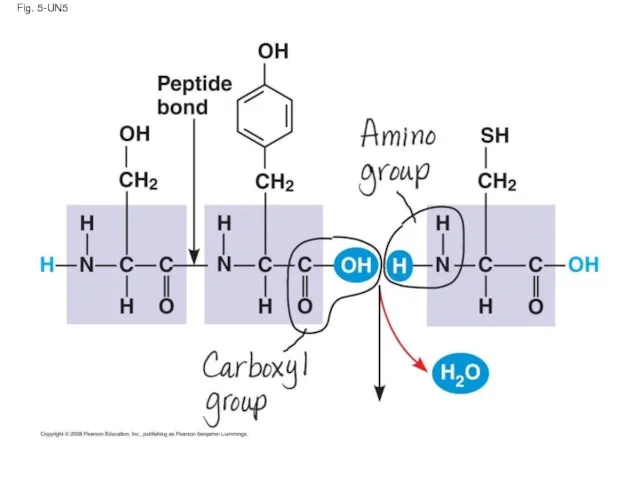
- 62. Fig. 5-UN1 Amino group Carboxyl group carbon
- 63. Fig. 5-17 Nonpolar Glycine (Gly or G) Alanine (Ala or A) Valine (Val or V) Leucine
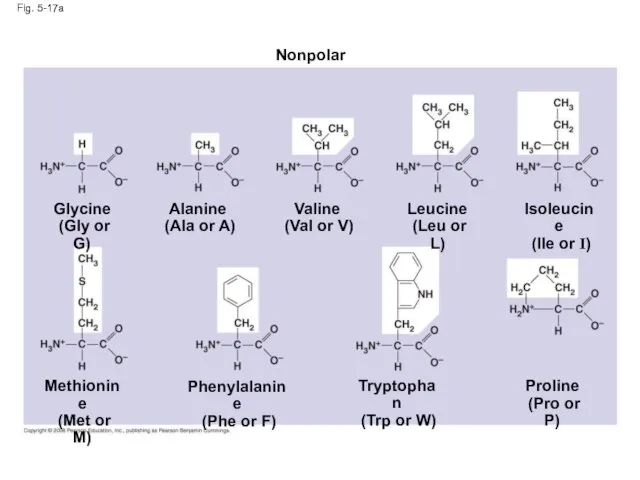
- 64. Fig. 5-17a Nonpolar Glycine (Gly or G) Alanine (Ala or A) Valine (Val or V) Leucine
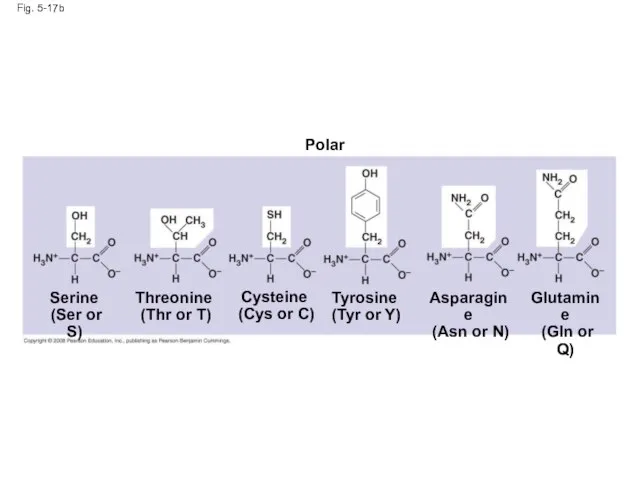
- 65. Fig. 5-17b Polar Asparagine (Asn or N) Glutamine (Gln or Q) Serine (Ser or S) Threonine
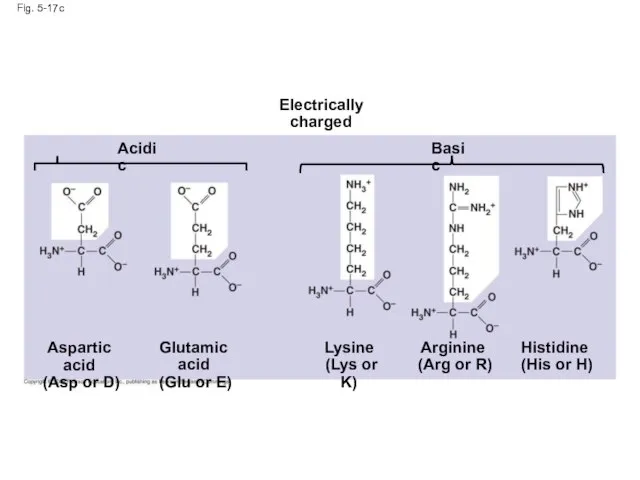
- 66. Fig. 5-17c Acidic Arginine (Arg or R) Histidine (His or H) Aspartic acid (Asp or D)
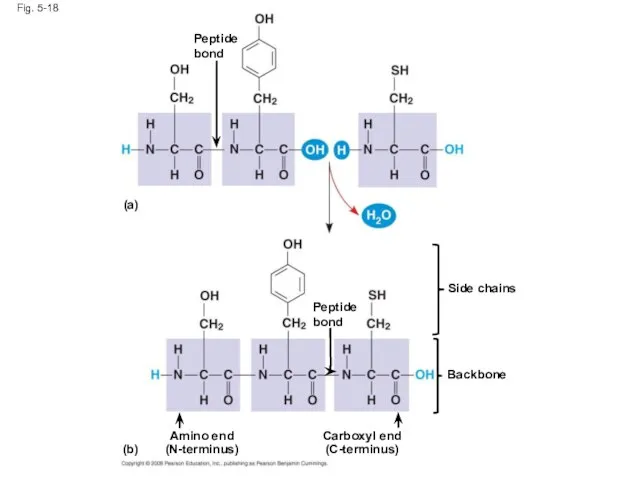
- 67. Amino Acid Polymers Amino acids are linked by peptide bonds A polypeptide is a polymer of
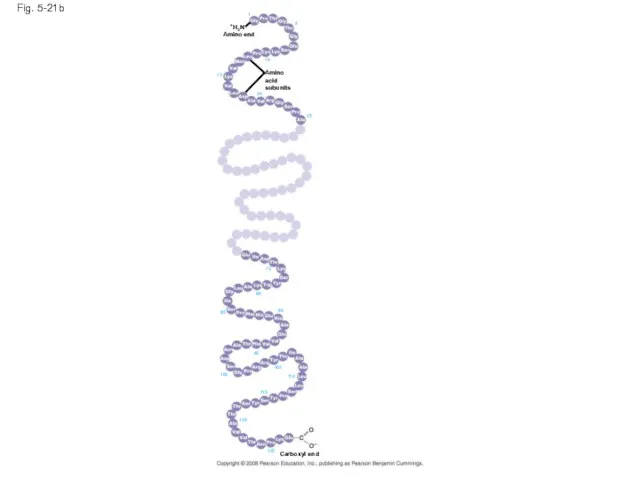
- 68. Peptide bond Fig. 5-18 Amino end (N-terminus) Peptide bond Side chains Backbone Carboxyl end (C-terminus) (a)
- 69. Protein Structure and Function A functional protein consists of one or more polypeptides twisted, folded, and
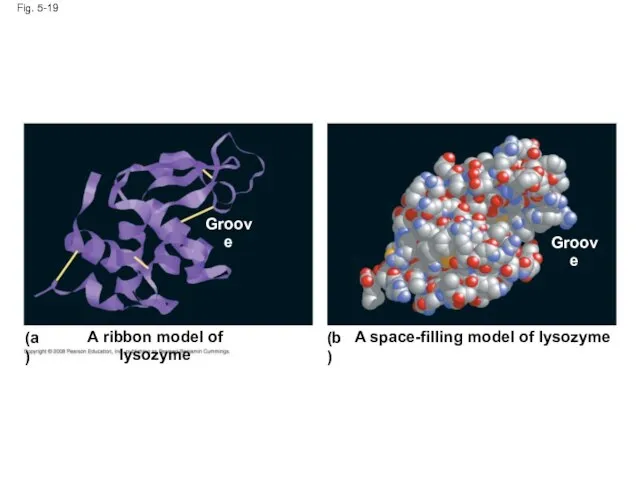
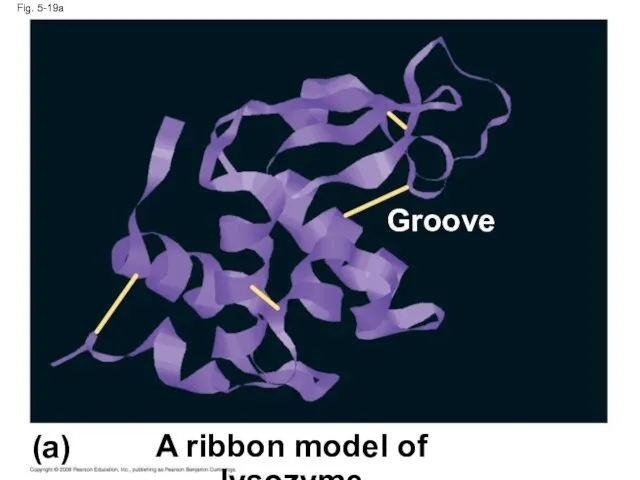
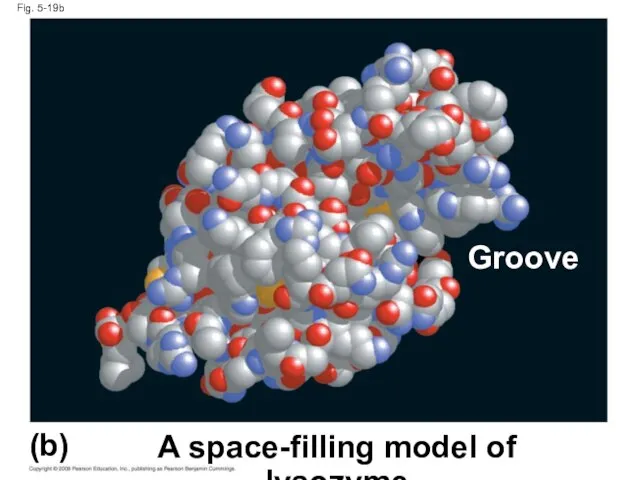
- 70. Fig. 5-19 A ribbon model of lysozyme (a) (b) A space-filling model of lysozyme Groove Groove
- 71. Fig. 5-19a A ribbon model of lysozyme (a) Groove
- 72. Fig. 5-19b (b) A space-filling model of lysozyme Groove
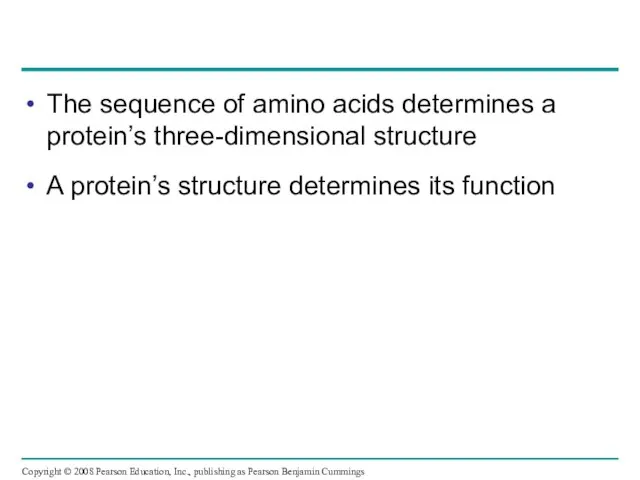
- 73. The sequence of amino acids determines a protein’s three-dimensional structure A protein’s structure determines its function
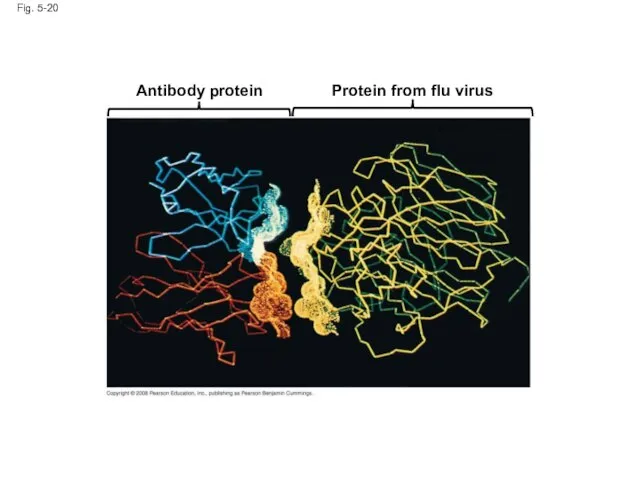
- 74. Fig. 5-20 Antibody protein Protein from flu virus
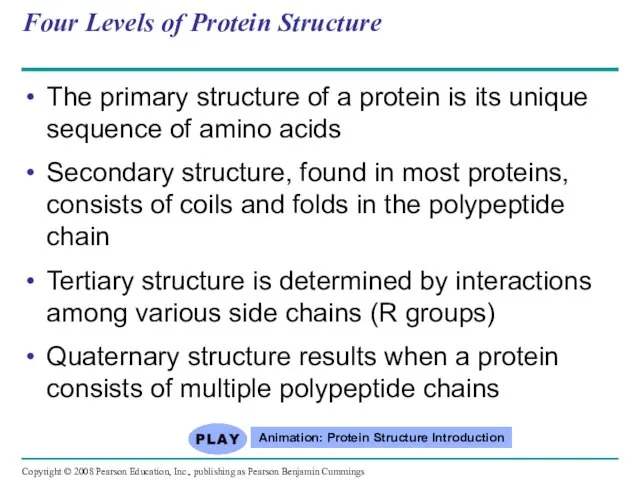
- 75. Four Levels of Protein Structure The primary structure of a protein is its unique sequence of
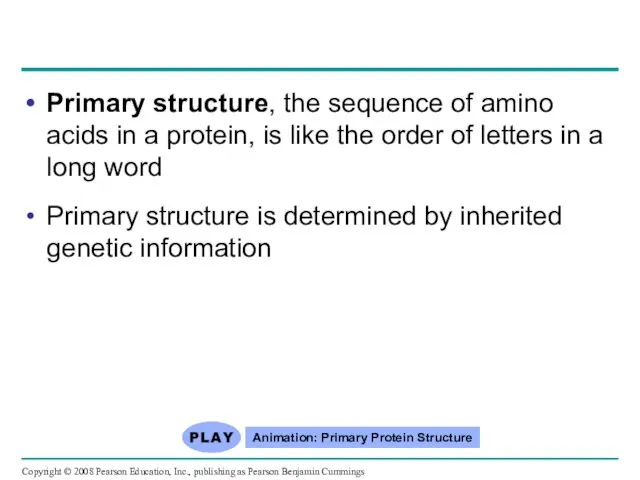
- 76. Primary structure, the sequence of amino acids in a protein, is like the order of letters
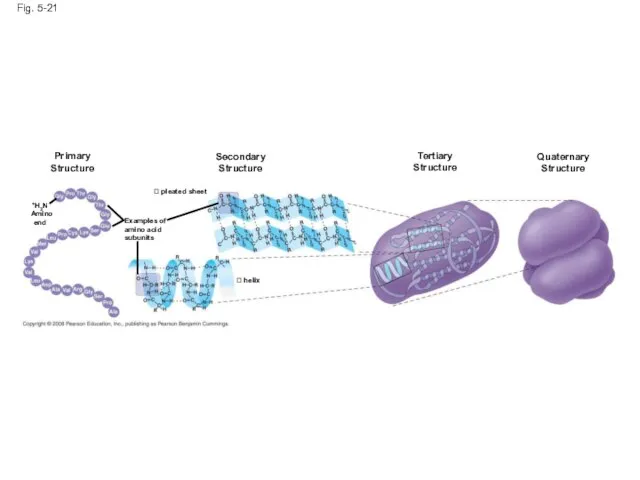
- 77. Fig. 5-21 Primary Structure Secondary Structure Tertiary Structure pleated sheet Examples of amino acid subunits
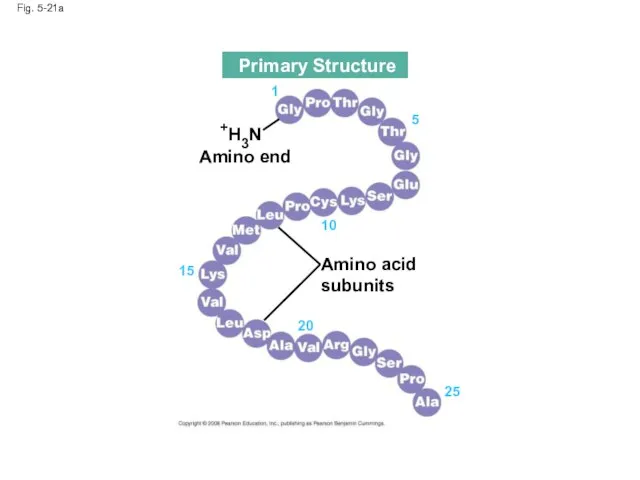
- 78. Fig. 5-21a Amino acid subunits +H3N Amino end 25 20 15 10 5 1 Primary Structure
- 79. Fig. 5-21b Amino acid subunits +H3N Amino end Carboxyl end 125 120 115 110 105 100
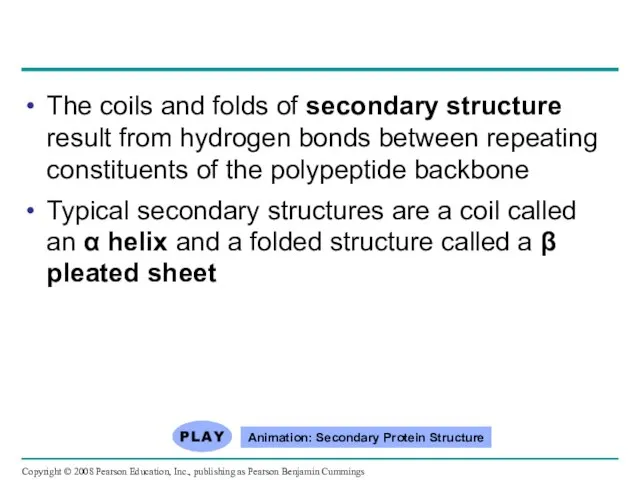
- 80. The coils and folds of secondary structure result from hydrogen bonds between repeating constituents of the
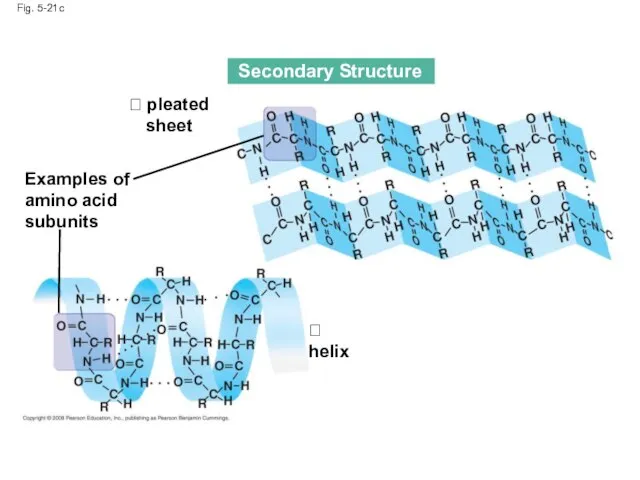
- 81. Fig. 5-21c Secondary Structure pleated sheet Examples of amino acid subunits helix
- 82. Fig. 5-21d Abdominal glands of the spider secrete silk fibers made of a structural protein containing
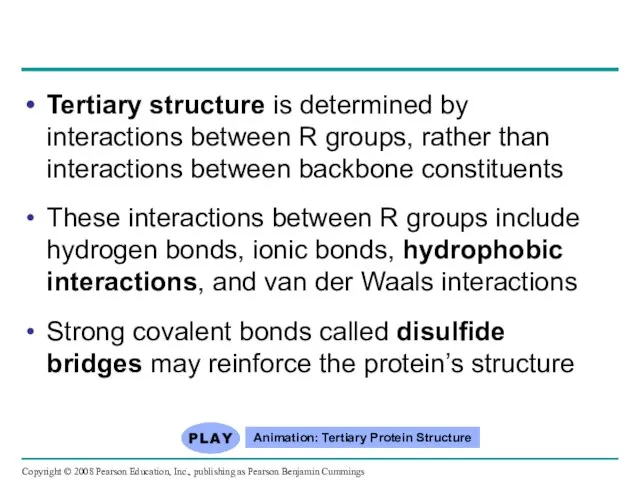
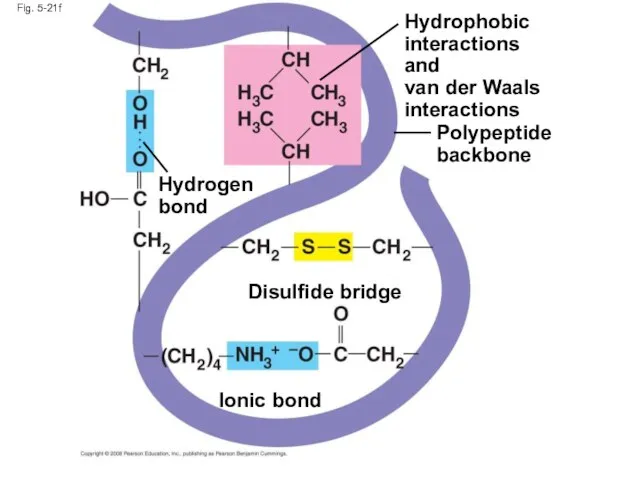
- 83. Tertiary structure is determined by interactions between R groups, rather than interactions between backbone constituents These
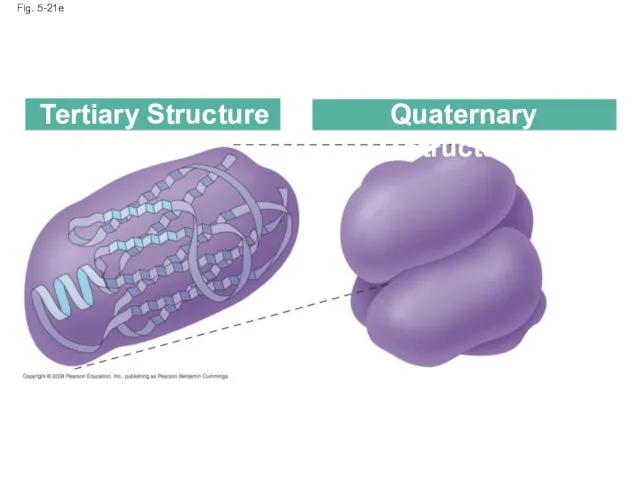
- 84. Fig. 5-21e Tertiary Structure Quaternary Structure
- 85. Fig. 5-21f Polypeptide backbone Hydrophobic interactions and van der Waals interactions Disulfide bridge Ionic bond Hydrogen
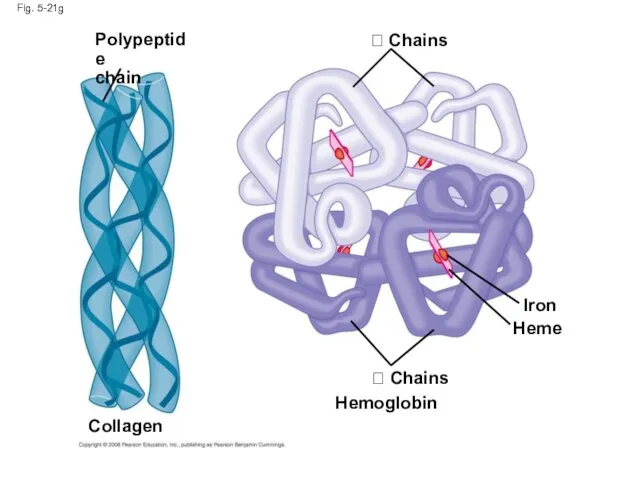
- 86. Fig. 5-21g Polypeptide chain Chains Heme Iron Chains Collagen Hemoglobin
- 87. Quaternary structure results when two or more polypeptide chains form one macromolecule Collagen is a fibrous
- 88. Sickle-Cell Disease: A Change in Primary Structure A slight change in primary structure can affect a
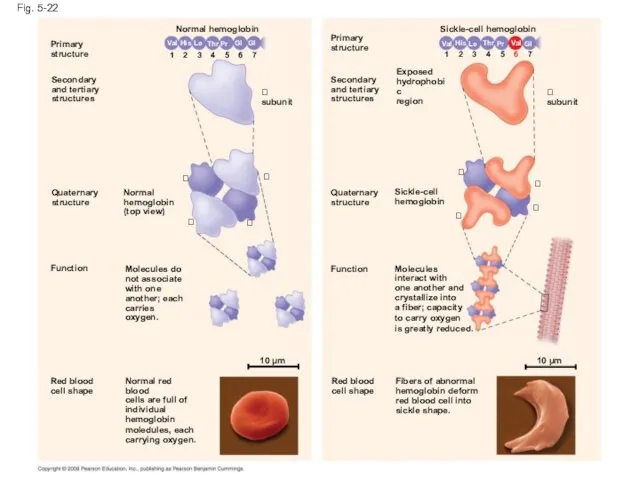
- 89. Fig. 5-22 Primary structure Secondary and tertiary structures Quaternary structure Normal hemoglobin (top view) Primary structure
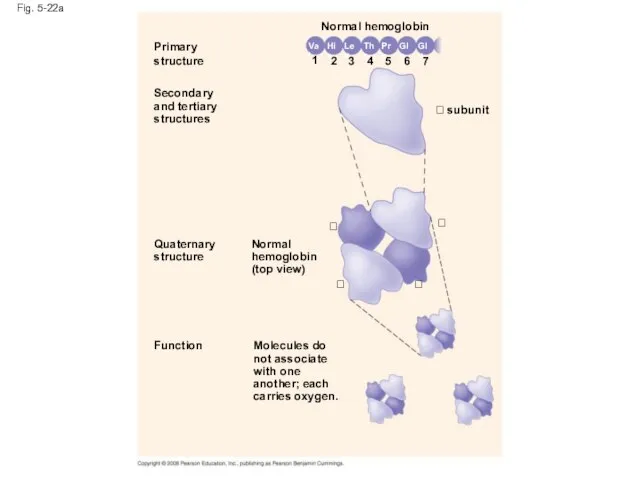
- 90. Fig. 5-22a Primary structure Secondary and tertiary structures Function Quaternary structure Molecules do not associate with
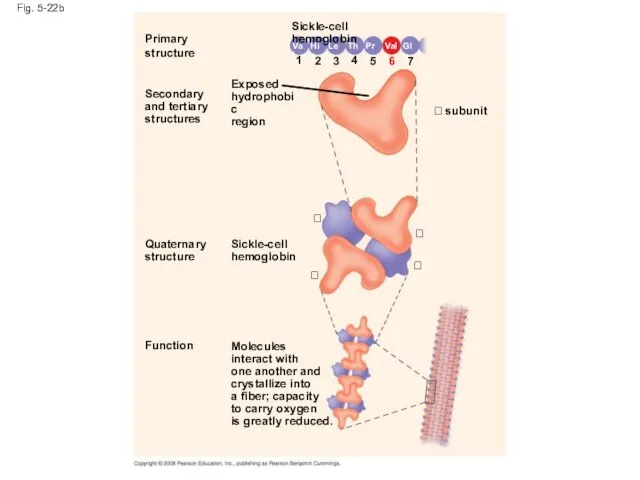
- 91. Fig. 5-22b Primary structure Secondary and tertiary structures Function Quaternary structure Molecules interact with one another
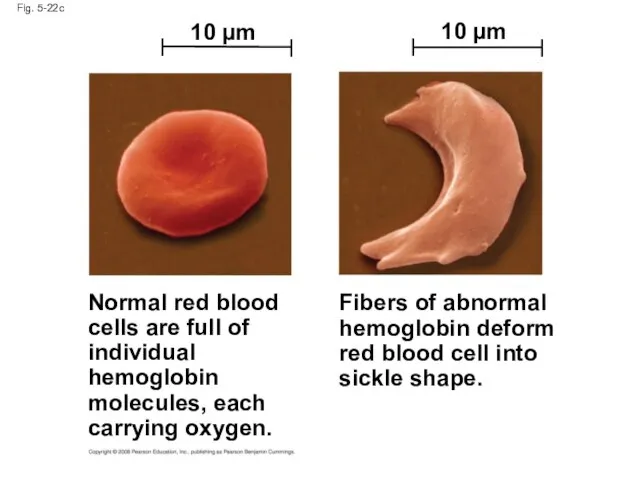
- 92. Fig. 5-22c Normal red blood cells are full of individual hemoglobin molecules, each carrying oxygen. Fibers
- 93. What Determines Protein Structure? In addition to primary structure, physical and chemical conditions can affect structure
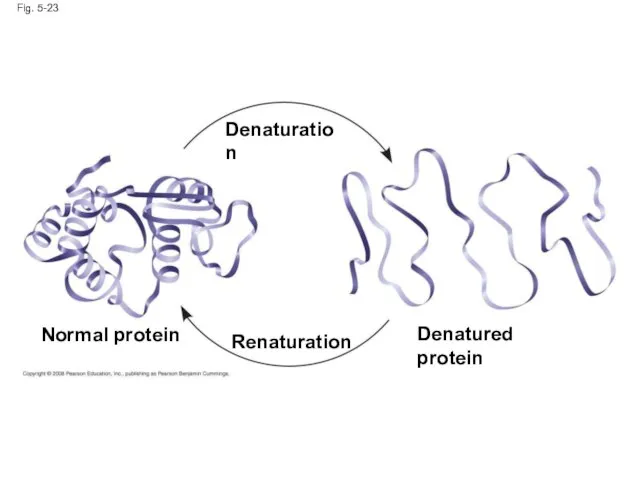
- 94. Fig. 5-23 Normal protein Denatured protein Denaturation Renaturation
- 95. Protein Folding in the Cell It is hard to predict a protein’s structure from its primary
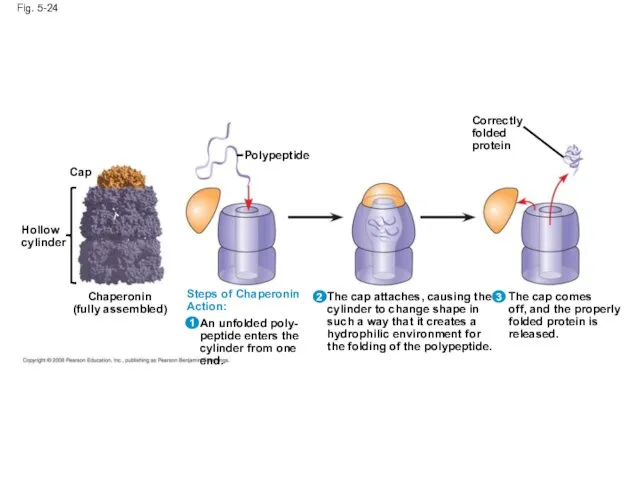
- 96. Fig. 5-24 Hollow cylinder Cap Chaperonin (fully assembled) Polypeptide Steps of Chaperonin Action: An unfolded poly-
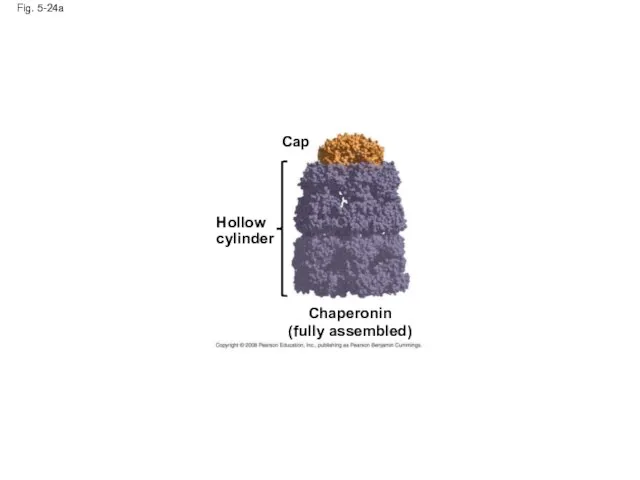
- 97. Fig. 5-24a Hollow cylinder Chaperonin (fully assembled) Cap
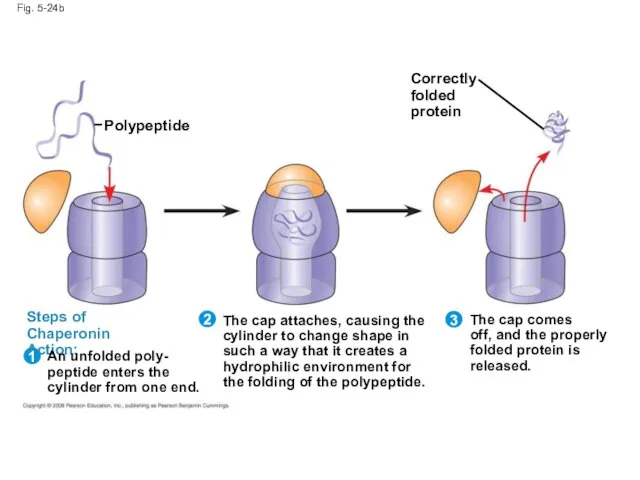
- 98. Fig. 5-24b Correctly folded protein Polypeptide Steps of Chaperonin Action: 1 2 An unfolded poly- peptide
- 99. Scientists use X-ray crystallography to determine a protein’s structure Another method is nuclear magnetic resonance (NMR)
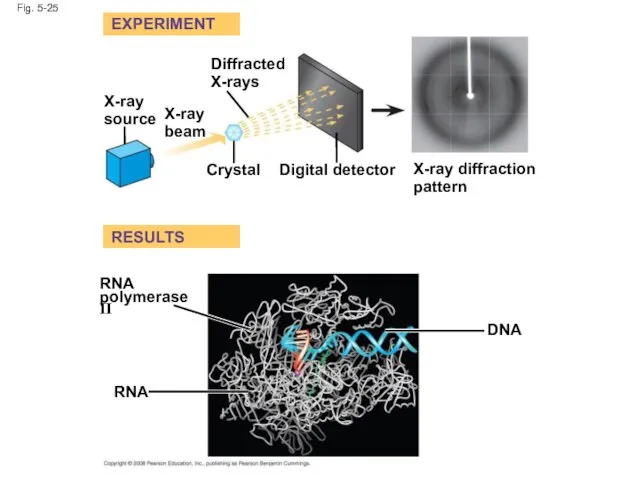
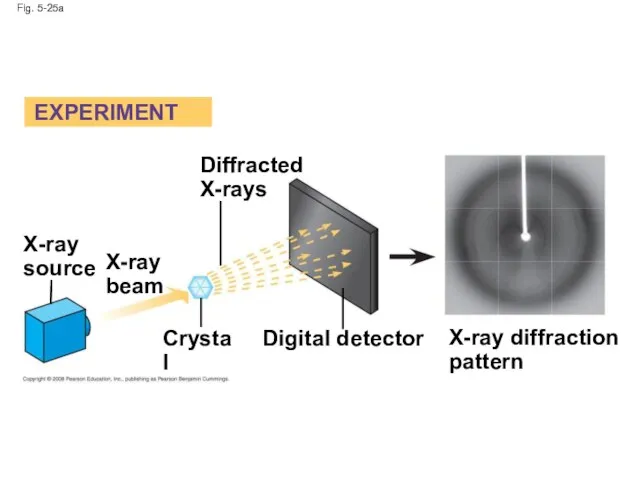
- 100. Fig. 5-25 EXPERIMENT RESULTS X-ray source X-ray beam Diffracted X-rays Crystal Digital detector X-ray diffraction pattern
- 101. Fig. 5-25a Diffracted X-rays EXPERIMENT X-ray source X-ray beam Crystal Digital detector X-ray diffraction pattern
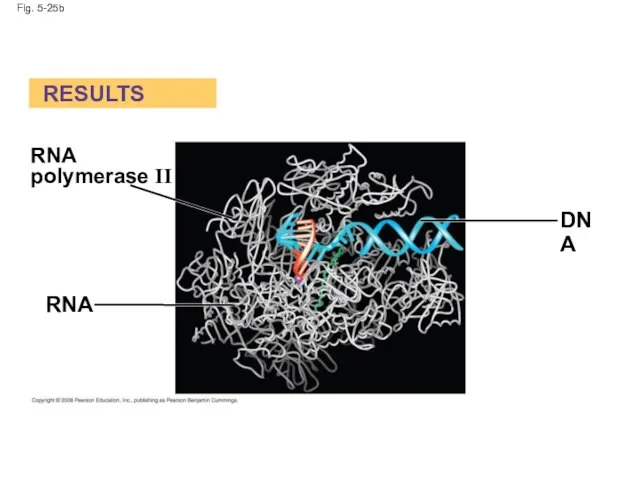
- 102. Fig. 5-25b RESULTS RNA RNA polymerase II DNA
- 103. Concept 5.5: Nucleic acids store and transmit hereditary information The amino acid sequence of a polypeptide
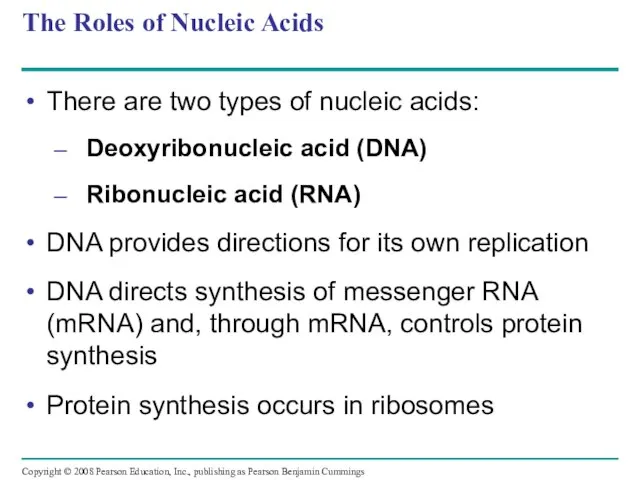
- 104. The Roles of Nucleic Acids There are two types of nucleic acids: Deoxyribonucleic acid (DNA) Ribonucleic
- 105. Fig. 5-26-1 mRNA Synthesis of mRNA in the nucleus DNA NUCLEUS CYTOPLASM 1
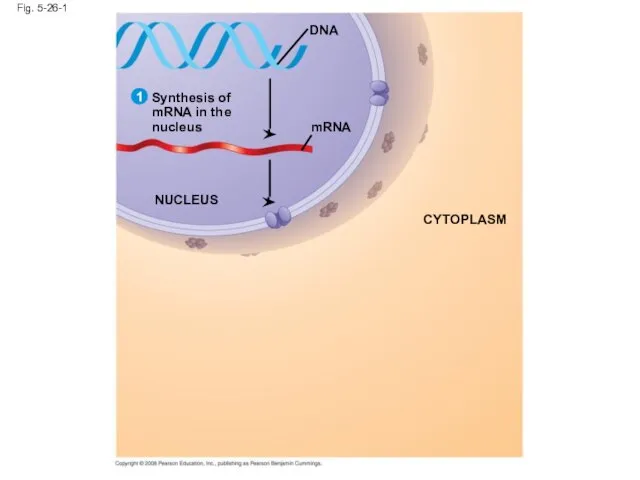
- 106. Fig. 5-26-2 mRNA Synthesis of mRNA in the nucleus DNA NUCLEUS mRNA CYTOPLASM Movement of mRNA
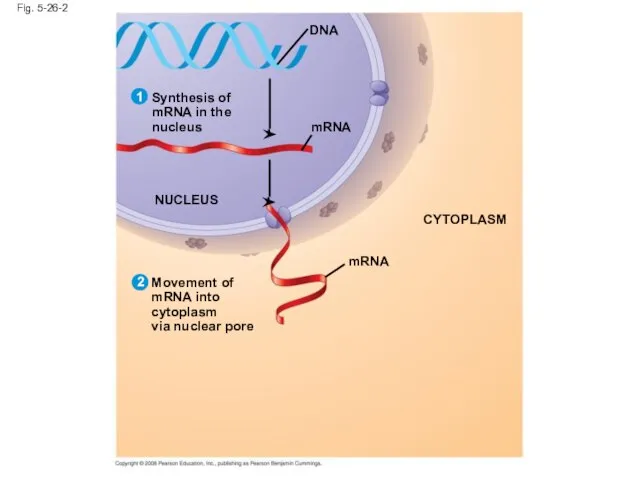
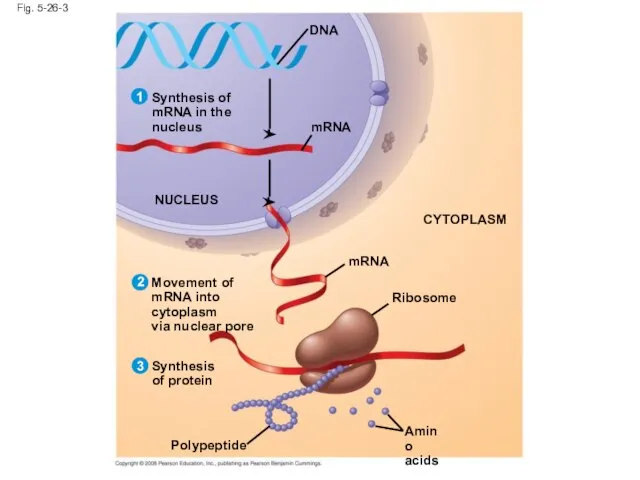
- 107. Fig. 5-26-3 mRNA Synthesis of mRNA in the nucleus DNA NUCLEUS mRNA CYTOPLASM Movement of mRNA
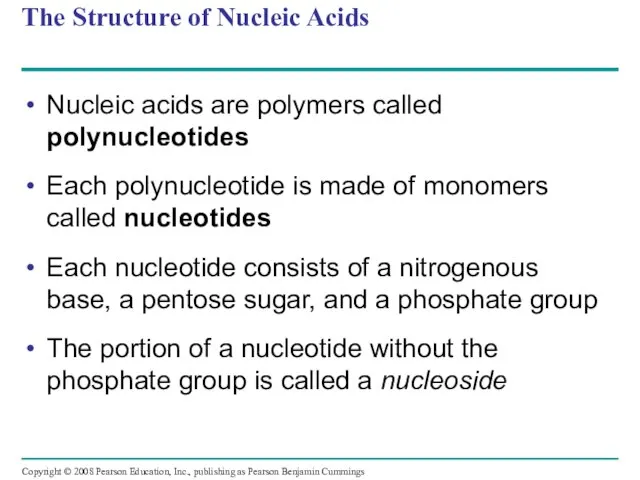
- 108. The Structure of Nucleic Acids Nucleic acids are polymers called polynucleotides Each polynucleotide is made of
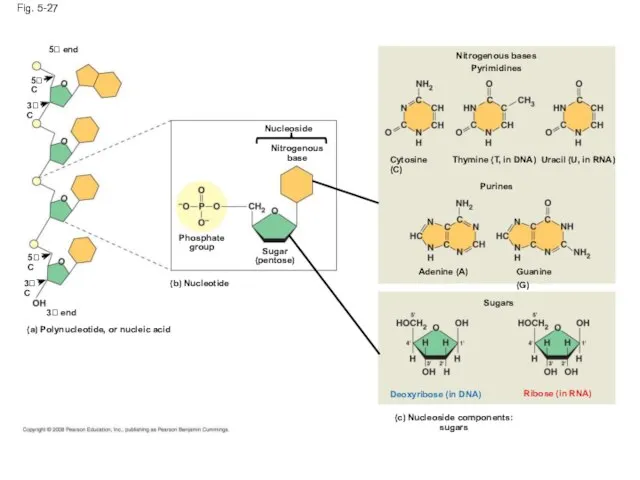
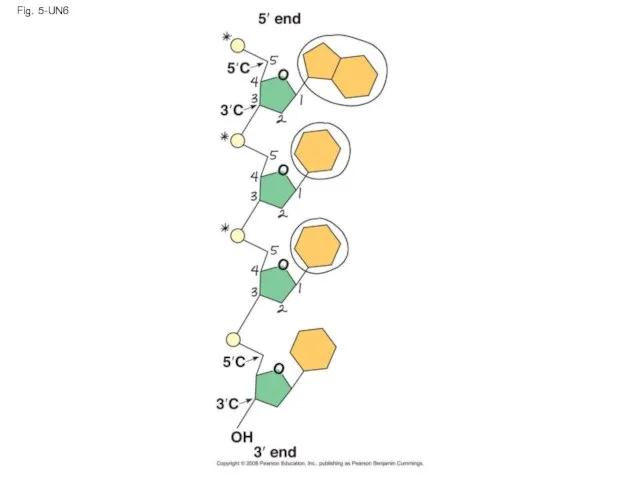
- 109. Fig. 5-27 5 end Nucleoside Nitrogenous base Phosphate group Sugar (pentose) (b) Nucleotide (a) Polynucleotide, or
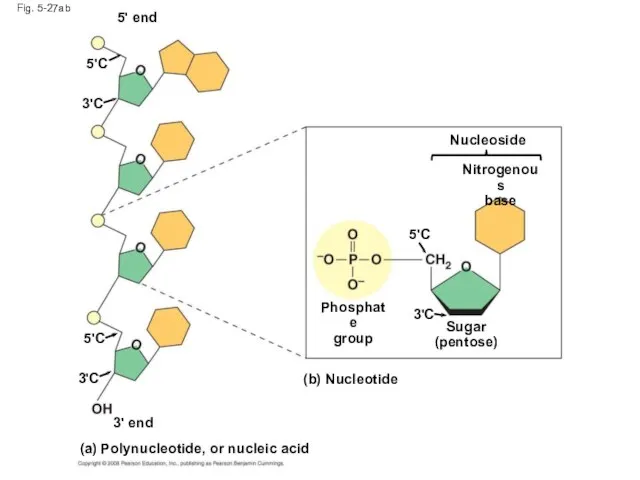
- 110. Fig. 5-27ab 5' end 5'C 3'C 5'C 3'C 3' end (a) Polynucleotide, or nucleic acid (b)
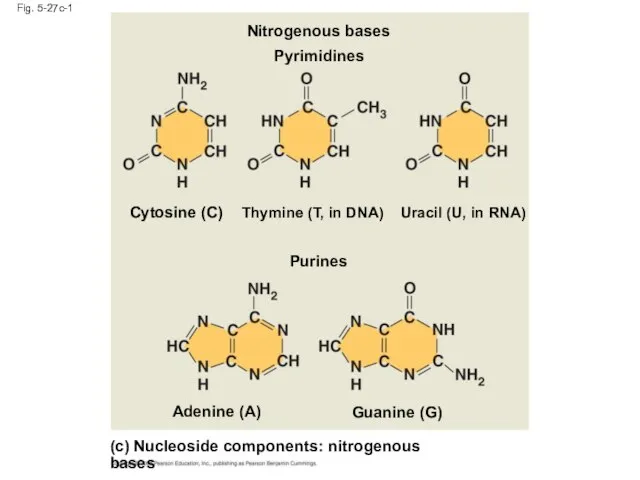
- 111. Fig. 5-27c-1 (c) Nucleoside components: nitrogenous bases Purines Guanine (G) Adenine (A) Cytosine (C) Thymine (T,
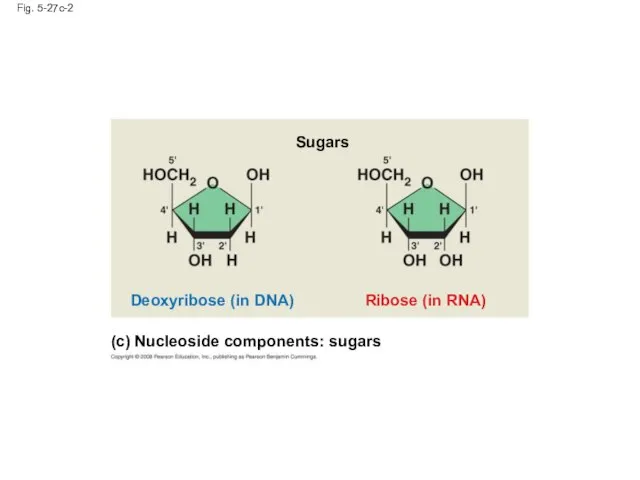
- 112. Fig. 5-27c-2 Ribose (in RNA) Deoxyribose (in DNA) Sugars (c) Nucleoside components: sugars
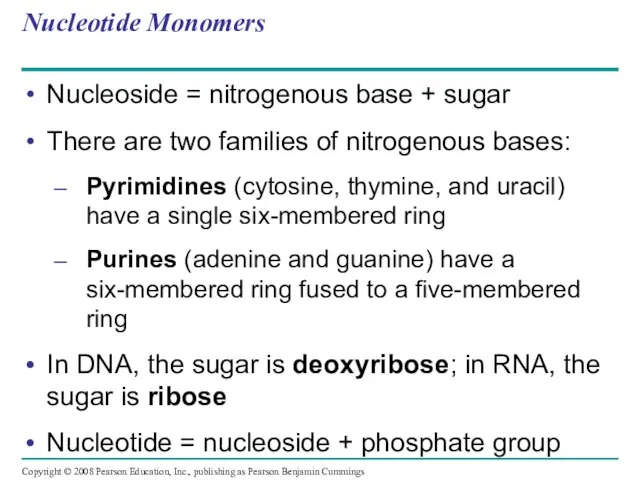
- 113. Nucleotide Monomers Nucleoside = nitrogenous base + sugar There are two families of nitrogenous bases: Pyrimidines
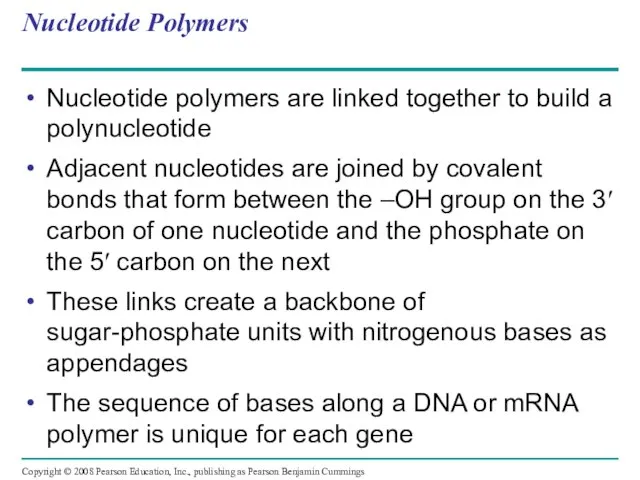
- 114. Nucleotide Polymers Nucleotide polymers are linked together to build a polynucleotide Adjacent nucleotides are joined by
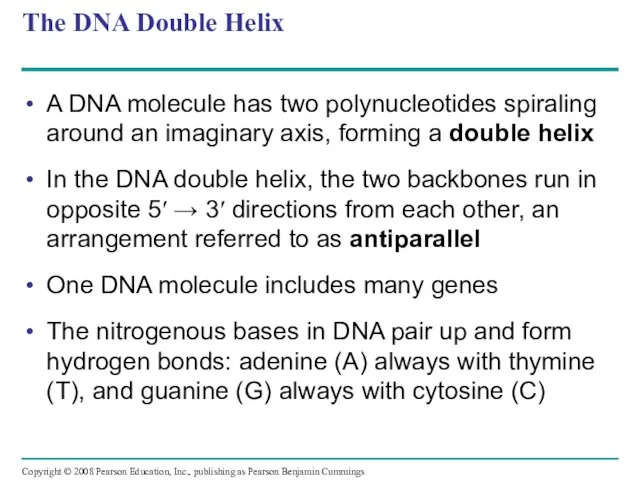
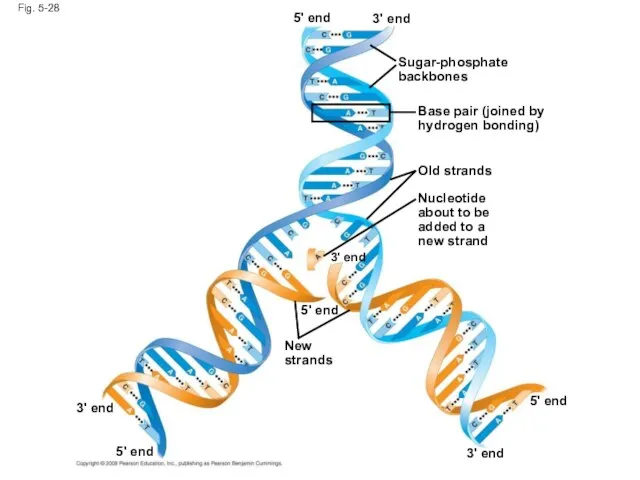
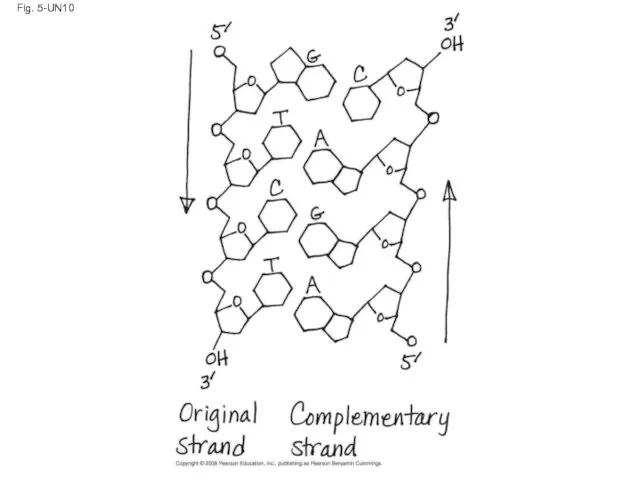
- 115. The DNA Double Helix A DNA molecule has two polynucleotides spiraling around an imaginary axis, forming
- 116. Fig. 5-28 Sugar-phosphate backbones 3' end 3' end 3' end 3' end 5' end 5' end
- 117. DNA and Proteins as Tape Measures of Evolution The linear sequences of nucleotides in DNA molecules
- 118. The Theme of Emergent Properties in the Chemistry of Life: A Review Higher levels of organization
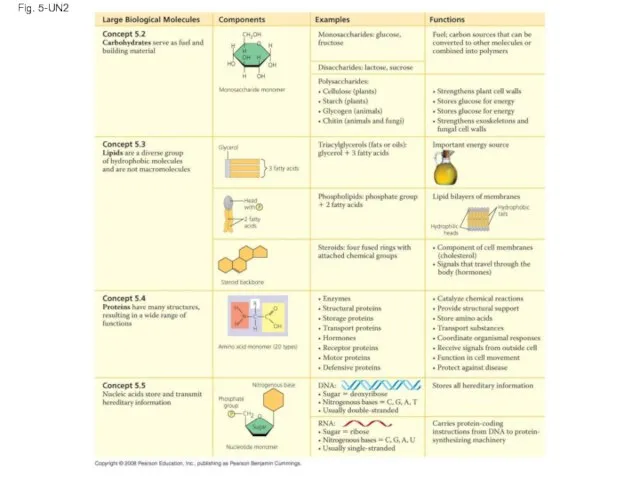
- 119. Fig. 5-UN2
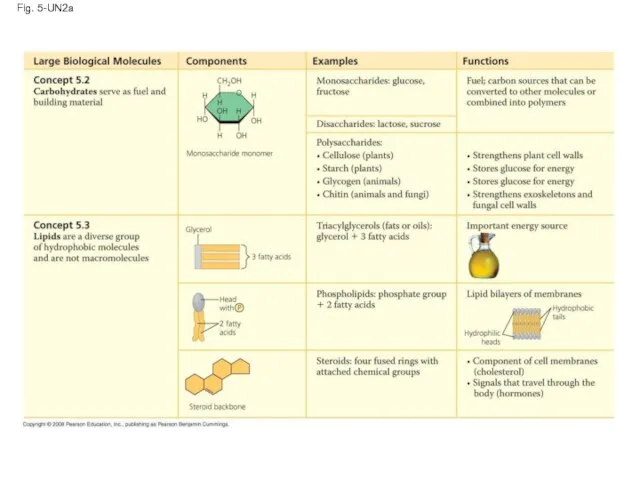
- 120. Fig. 5-UN2a
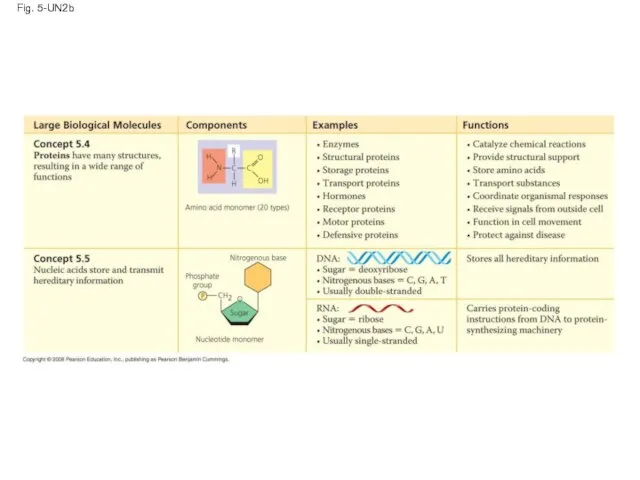
- 121. Fig. 5-UN2b
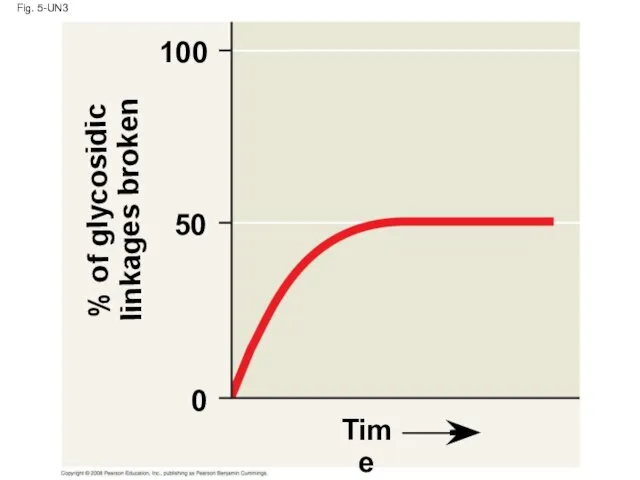
- 122. Fig. 5-UN3 % of glycosidic linkages broken 100 50 0 Time
- 123. Fig. 5-UN4
- 124. Fig. 5-UN5
- 125. Fig. 5-UN6
- 126. Fig. 5-UN7
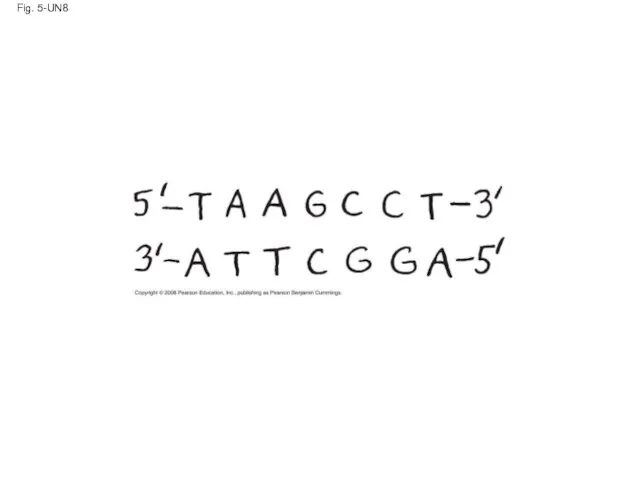
- 127. Fig. 5-UN8
- 128. Fig. 5-UN9
- 129. Fig. 5-UN10
- 130. You should now be able to: List and describe the four major classes of molecules Describe
- 132. Скачать презентацию










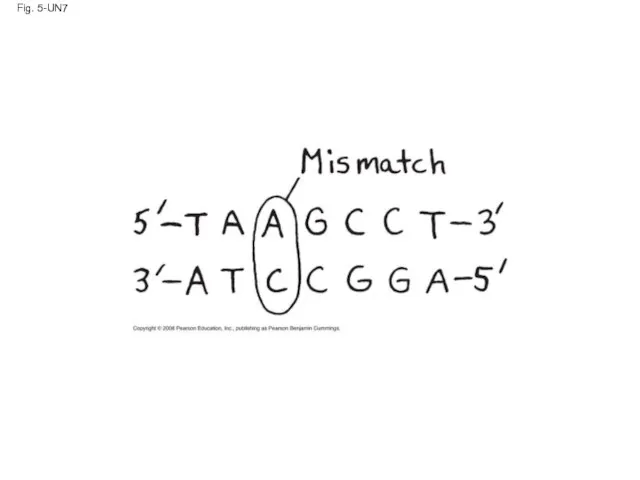
 Тип Плоские черви Презентация для 7 класса
Тип Плоские черви Презентация для 7 класса Презентация на тему Отряд Жуки или Жесткокрылые
Презентация на тему Отряд Жуки или Жесткокрылые  ТЕМА: «Класс рыб» Автор Самойленко Э.А., учитель биологии.
ТЕМА: «Класс рыб» Автор Самойленко Э.А., учитель биологии.  Сон
Сон Этапы формирования и развития представлений о клетке
Этапы формирования и развития представлений о клетке Автор: учитель биологии высшей категории МОУ «Засосенская СОШ имени Героя Советского Союза Н.Л.Яценко» Ковшов Анатолий Владимир
Автор: учитель биологии высшей категории МОУ «Засосенская СОШ имени Героя Советского Союза Н.Л.Яценко» Ковшов Анатолий Владимир Обмен белков
Обмен белков Профілактика інфекційних захворювань Бартош Наталії 11-А клас
Профілактика інфекційних захворювань Бартош Наталії 11-А клас  Тип Хордовые класс Млекопитающие Спесивцева О.А.
Тип Хордовые класс Млекопитающие Спесивцева О.А. Опорно-двигательная система, филогенез
Опорно-двигательная система, филогенез Отряд гусеобразные
Отряд гусеобразные Урок литературного чтения в 4 классе Автор: учитель начальных классов МБОУ СОШ № 6 Федченко Валентина Николаевна
Урок литературного чтения в 4 классе Автор: учитель начальных классов МБОУ СОШ № 6 Федченко Валентина Николаевна  Зерновые культуры
Зерновые культуры Прикладная анатомия гортани
Прикладная анатомия гортани Секвестрэктомия у пустынного канюка
Секвестрэктомия у пустынного канюка Презентация на тему "Вода – растворитель" - скачать презентации по Биологии
Презентация на тему "Вода – растворитель" - скачать презентации по Биологии Синичкин день
Синичкин день Органические вещества, входящие в состав клетки
Органические вещества, входящие в состав клетки Санитарная микробиология
Санитарная микробиология Презентация на тему В Арктике. Растительный и животный мир Арктики.
Презентация на тему В Арктике. Растительный и животный мир Арктики. Самые удивительные растения мира Растительный мир очень красив и многообразен. Сегодня существует около 360.000 различных видов
Самые удивительные растения мира Растительный мир очень красив и многообразен. Сегодня существует около 360.000 различных видов  Будова й значення дихальної системи
Будова й значення дихальної системи Нуклеиновые кислоты
Нуклеиновые кислоты Физиология желчного пузыря
Физиология желчного пузыря Разнообразие животных
Разнообразие животных Мейоз. Механизм мейоза
Мейоз. Механизм мейоза Программированный опрос по биологии
Программированный опрос по биологии Какие бывают животные
Какие бывают животные