Содержание
- 2. Acids and alkalis Solutions can be sorted by whether they are: acid, alkali or neutral. When
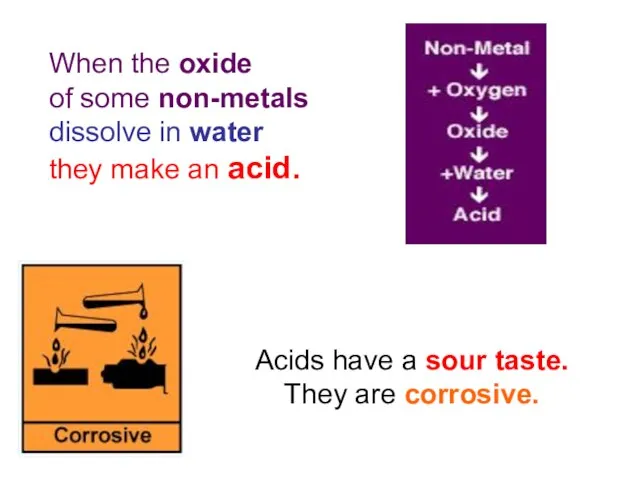
- 3. When the oxide of some non-metals dissolve in water they make an acid. Acids have a
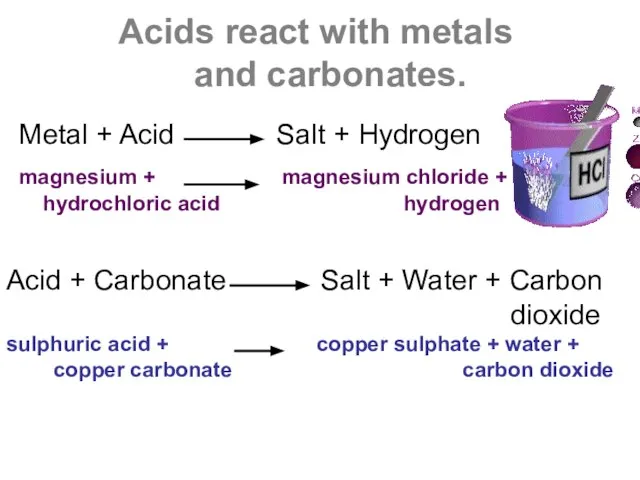
- 4. Acids react with metals and carbonates. Metal + Acid Salt + Hydrogen magnesium + magnesium chloride
- 5. Acids Lemon juice contains citric acid, and vinegar contains ethanoic acid. Some strong acids are hydrochloric
- 6. Neutralisation Acids and alkalis react with each other. The alkali cancels out the acid in the
- 7. Salts The salt made depends on the acid and alkali used. The salt contains the metal
- 8. Alkalis When the oxides of some metals dissolve in water they make an alkali solution. Alkalis
- 9. Alkalis Alkalis are present in many cleaning substances in use in our homes. Kitchen cleaners are
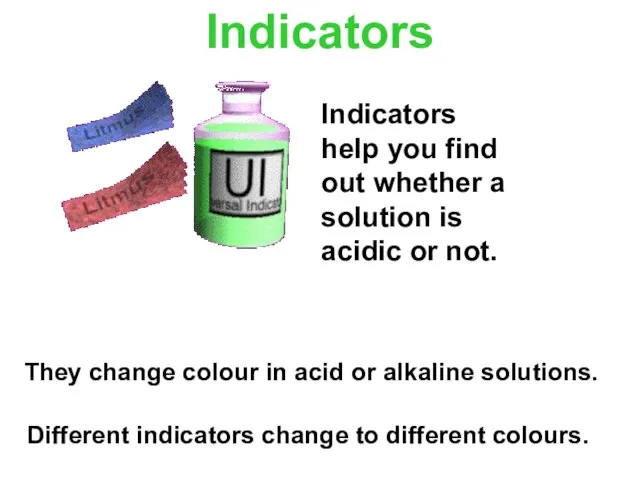
- 10. Indicators They change colour in acid or alkaline solutions. Different indicators change to different colours. Indicators
- 11. Litmus Test Litmus is an indicator. It changes colour in acid and alkaline solutions. Litmus is
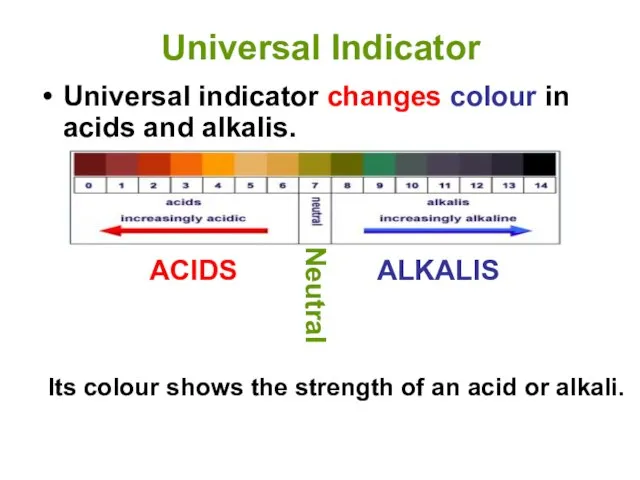
- 12. Universal Indicator Universal indicator changes colour in acids and alkalis. Its colour shows the strength of
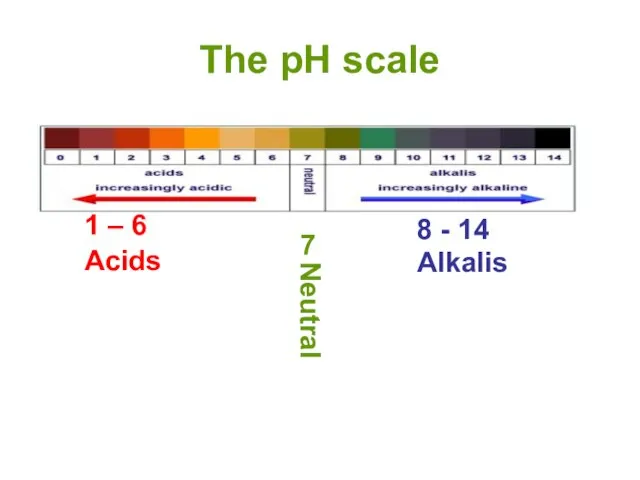
- 13. The pH scale 1 – 6 8 - 14 Alkalis 7 Neutral Acids
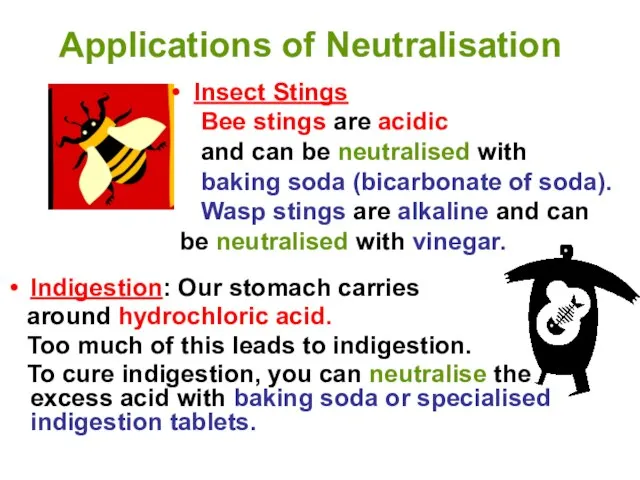
- 14. Applications of Neutralisation Indigestion: Our stomach carries around hydrochloric acid. Too much of this leads to
- 16. Скачать презентацию







 Подготовка к ЕГЭ: Кислородсодержащие органические соединения. КАРБОНОВЫЕ КИСЛОТЫ. Муниципальное бюджетное общеобразовательное
Подготовка к ЕГЭ: Кислородсодержащие органические соединения. КАРБОНОВЫЕ КИСЛОТЫ. Муниципальное бюджетное общеобразовательное  Охрана почв при применении пестицидов
Охрана почв при применении пестицидов Метаболизм липидов. Характеристика липидов. Значение. Представители. Эйкозаноиды. (Лекция 1-2)
Метаболизм липидов. Характеристика липидов. Значение. Представители. Эйкозаноиды. (Лекция 1-2) Узинская ООШ исслед.работа Лобановой Елизаветы
Узинская ООШ исслед.работа Лобановой Елизаветы Алканы: физические и химические свойства, получение
Алканы: физические и химические свойства, получение Презентация по Химии "Кислород" - скачать смотреть _
Презентация по Химии "Кислород" - скачать смотреть _ Азотная кислота. Состав. Строение. Физические свойства
Азотная кислота. Состав. Строение. Физические свойства Зелёная химия
Зелёная химия Железо как химический элемент
Железо как химический элемент Общая и неорганическая химия
Общая и неорганическая химия Адсорбционные равновесия. Межмолекулярные взаимодействия при адсорбции
Адсорбционные равновесия. Межмолекулярные взаимодействия при адсорбции Электродные потенциалы. Окислительно-восстановительные потенциалы. Потенциометрия в медицинской практике
Электродные потенциалы. Окислительно-восстановительные потенциалы. Потенциометрия в медицинской практике Углерод и его соединения
Углерод и его соединения Анализ стадии технологического процесса производства гранулотола
Анализ стадии технологического процесса производства гранулотола Аттестационная работа. Исследование электрических свойств воды и её растворов
Аттестационная работа. Исследование электрических свойств воды и её растворов Презентация по Химии "Алмаз" - скачать смотреть бесплатно
Презентация по Химии "Алмаз" - скачать смотреть бесплатно Литье под давлением термопластов
Литье под давлением термопластов Нитрид индия – новый материал для оптоэлектроники
Нитрид индия – новый материал для оптоэлектроники Литосфера. Физико-химические процессы в литосфере
Литосфера. Физико-химические процессы в литосфере Количество вещества, число Авогадро, молярная масса, молярный объём, уравнение связи
Количество вещества, число Авогадро, молярная масса, молярный объём, уравнение связи Ионообменная хроматография
Ионообменная хроматография Atomic structure. Introduction
Atomic structure. Introduction Пластмаси, синтетичні каучуки Виконала: Учениця 11 – А класу Твердохліб Анжеліка
Пластмаси, синтетичні каучуки Виконала: Учениця 11 – А класу Твердохліб Анжеліка  Периодический закон и ПСХЭ Д.И. Менделеева в свете учения о строении атома
Периодический закон и ПСХЭ Д.И. Менделеева в свете учения о строении атома Щелочные металлы
Щелочные металлы Серная кислота H2SO4
Серная кислота H2SO4 ДЕЙТЕРИЙ. ТЯЖЕЛАЯ ВОДА
ДЕЙТЕРИЙ. ТЯЖЕЛАЯ ВОДА Бензол и его свойства
Бензол и его свойства