Содержание
- 2. ATOMIC STRUCTURE INTRODUCTION This Powerpoint show is one of several produced to help students understand selected
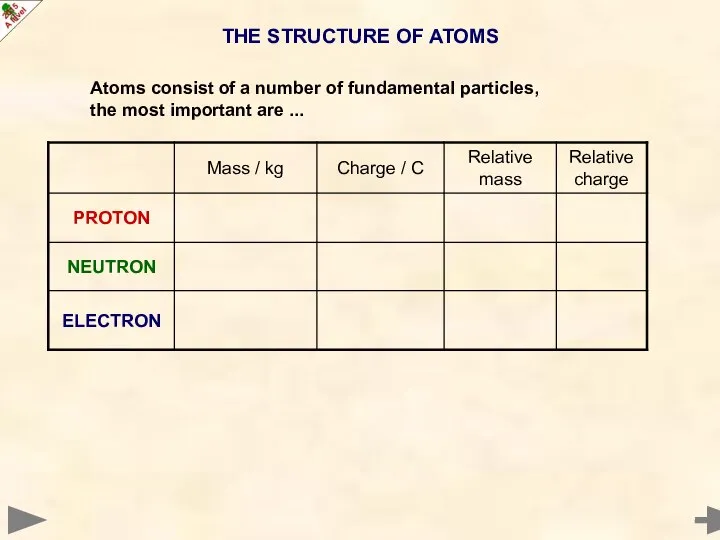
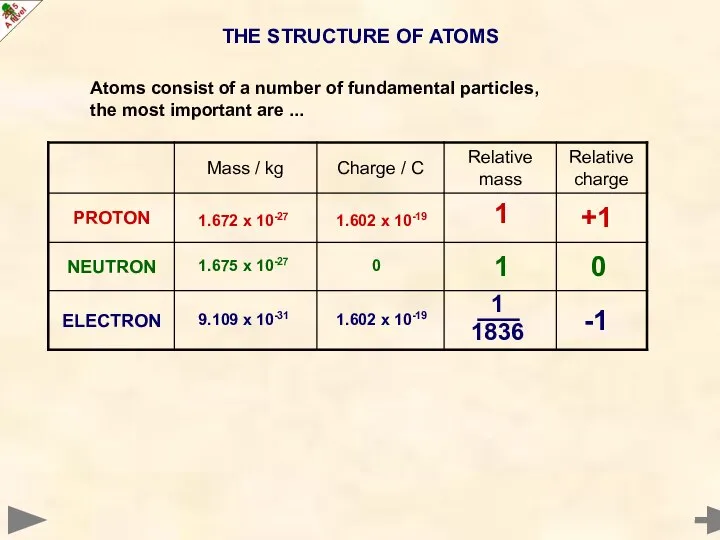
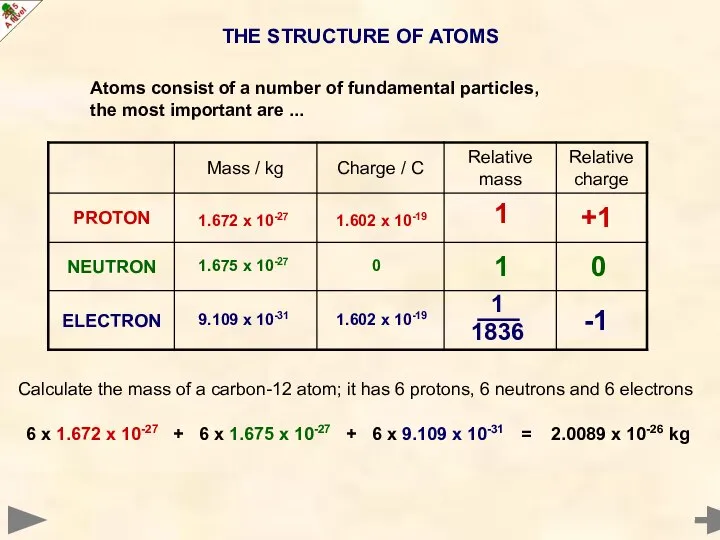
- 3. THE STRUCTURE OF ATOMS Atoms consist of a number of fundamental particles, the most important are
- 4. THE STRUCTURE OF ATOMS 0 -1 +1 1 1 1836 1 9.109 x 10-31 1.602 x
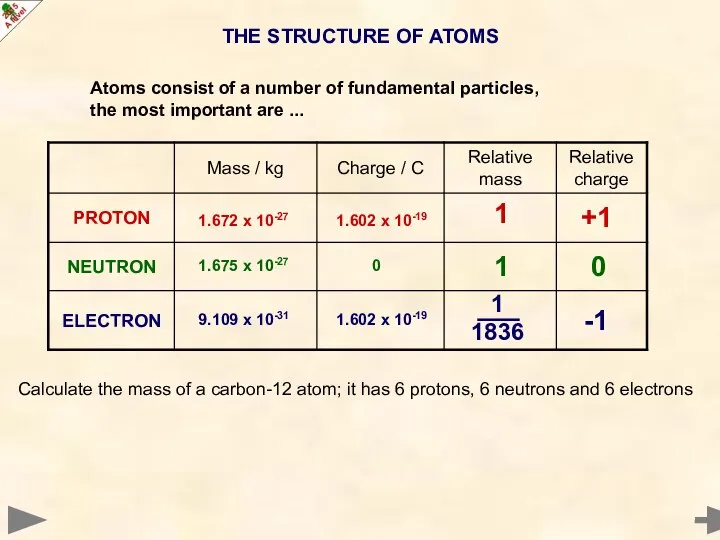
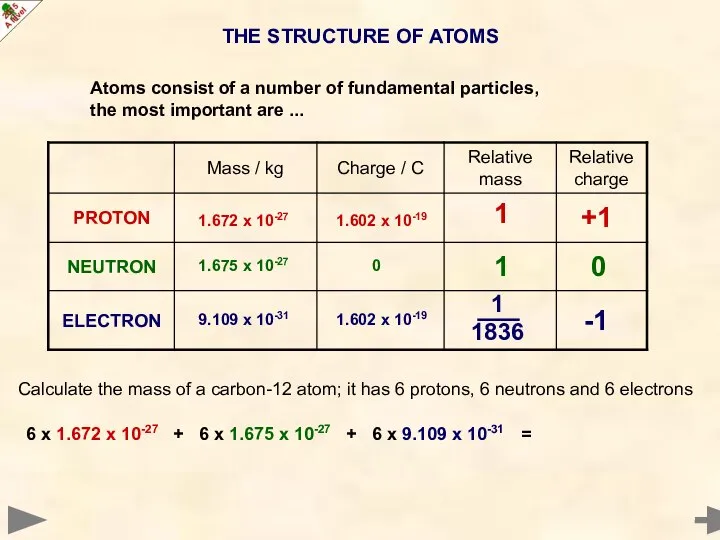
- 5. THE STRUCTURE OF ATOMS 0 -1 +1 1 1 1836 1 Calculate the mass of a
- 6. THE STRUCTURE OF ATOMS 0 -1 +1 1 1 1836 1 Calculate the mass of a
- 7. THE STRUCTURE OF ATOMS 0 -1 +1 1 1 1836 1 Calculate the mass of a
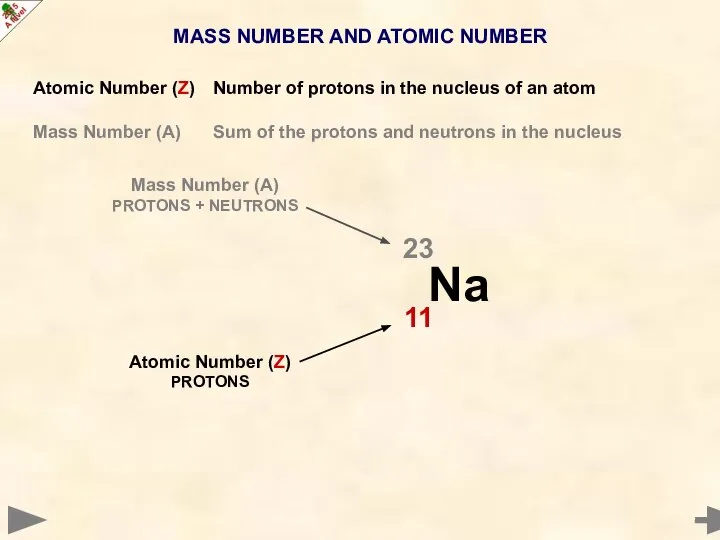
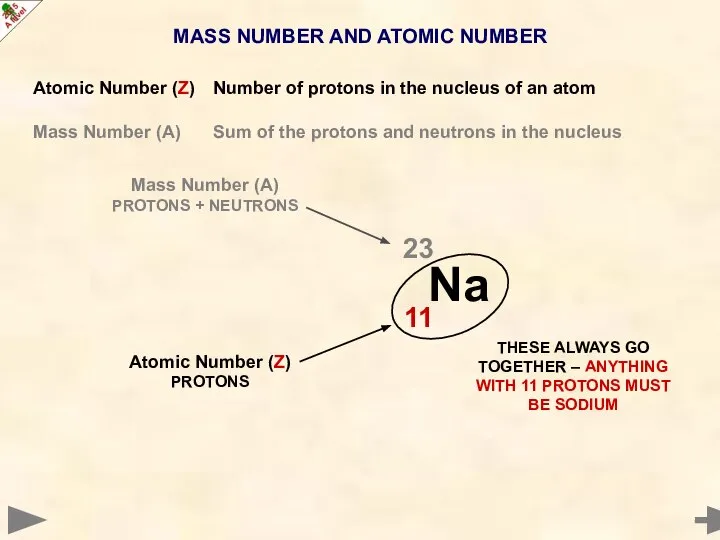
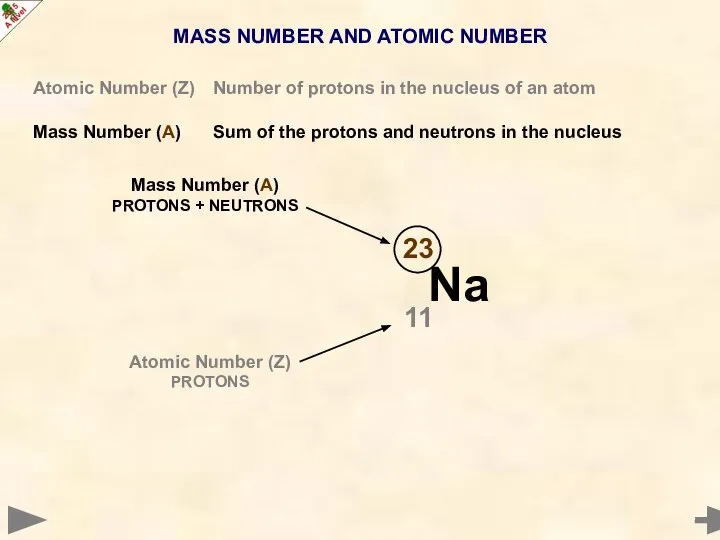
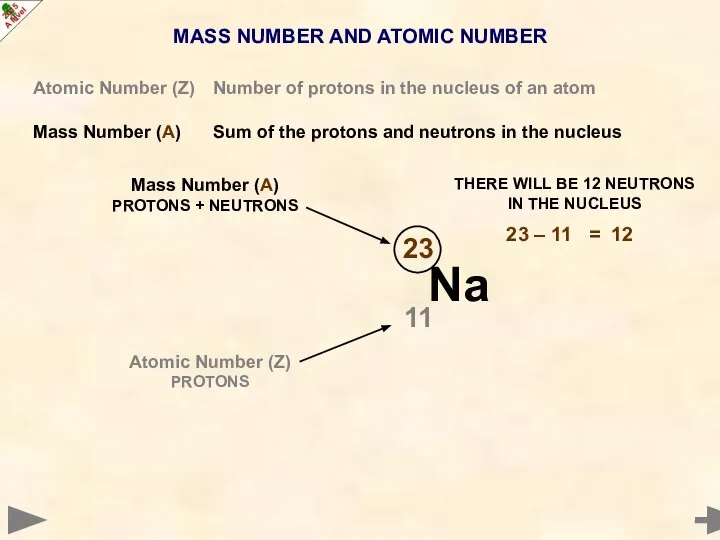
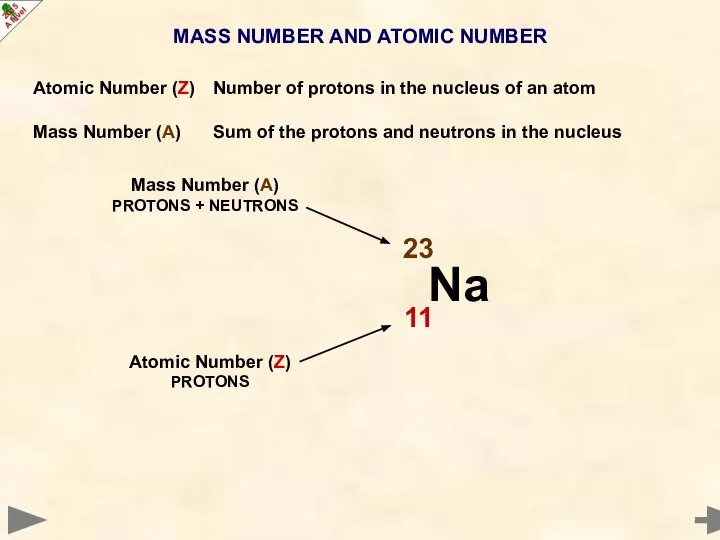
- 8. MASS NUMBER AND ATOMIC NUMBER Atomic Number (Z) Number of protons in the nucleus of an
- 9. MASS NUMBER AND ATOMIC NUMBER Atomic Number (Z) Number of protons in the nucleus of an
- 10. MASS NUMBER AND ATOMIC NUMBER Atomic Number (Z) Number of protons in the nucleus of an
- 11. MASS NUMBER AND ATOMIC NUMBER Atomic Number (Z) Number of protons in the nucleus of an
- 12. MASS NUMBER AND ATOMIC NUMBER Atomic Number (Z) Number of protons in the nucleus of an
- 13. MASS NUMBER AND ATOMIC NUMBER Atomic Number (Z) Number of protons in the nucleus of an
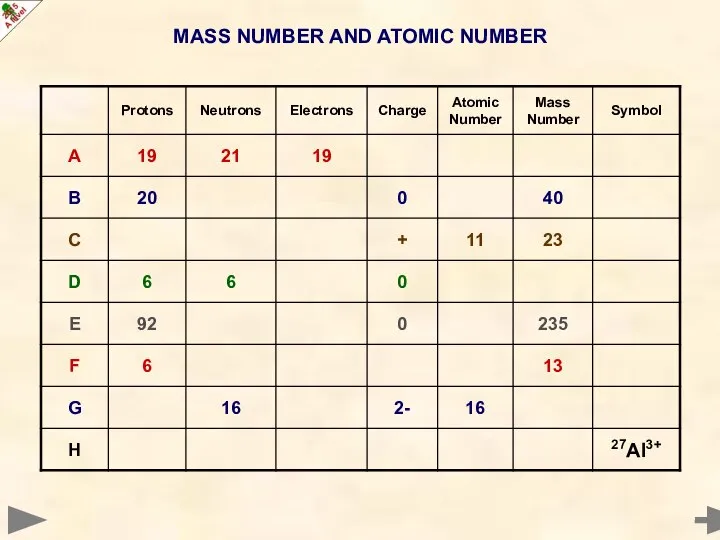
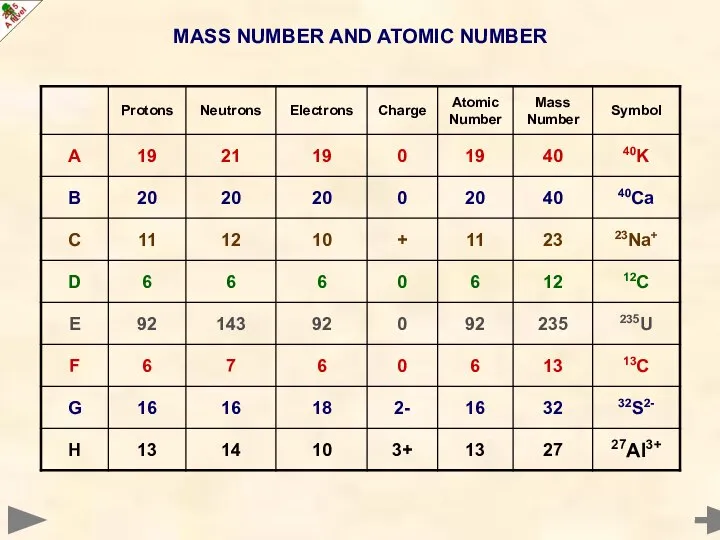
- 14. MASS NUMBER AND ATOMIC NUMBER
- 15. MASS NUMBER AND ATOMIC NUMBER
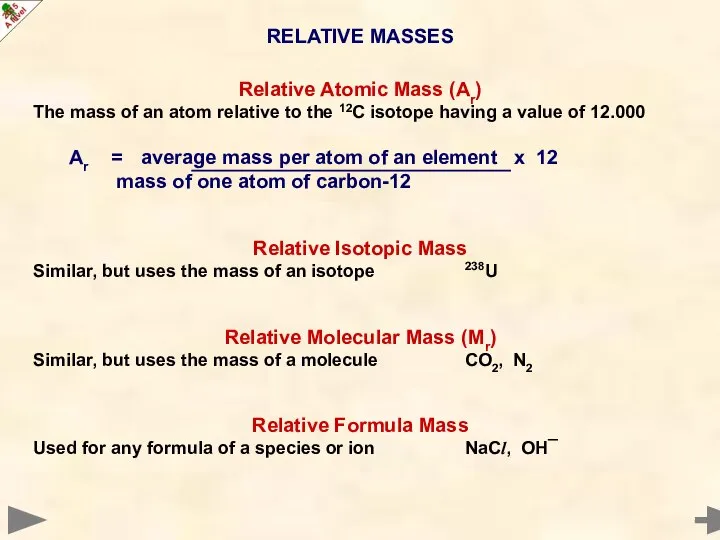
- 16. RELATIVE MASSES Relative Atomic Mass (Ar) The mass of an atom relative to the 12C isotope
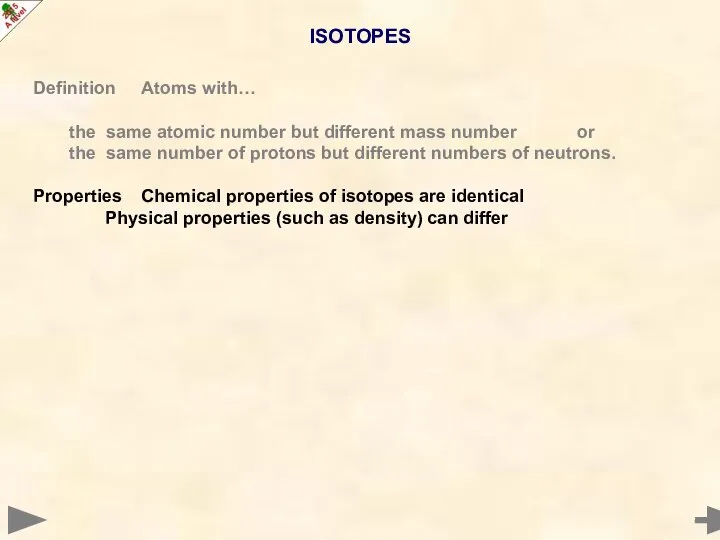
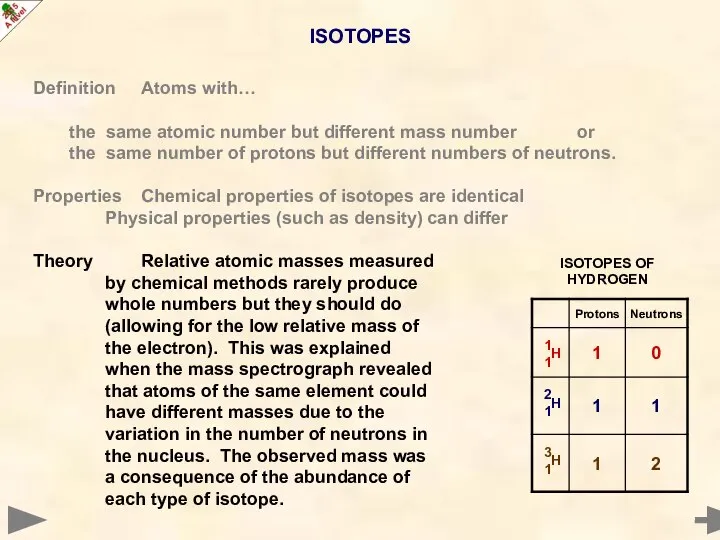
- 17. ISOTOPES Definition Atoms with… the same atomic number but different mass number or the same number
- 18. ISOTOPES Definition Atoms with… the same atomic number but different mass number or the same number
- 19. ISOTOPES Definition Atoms with… the same atomic number but different mass number or the same number
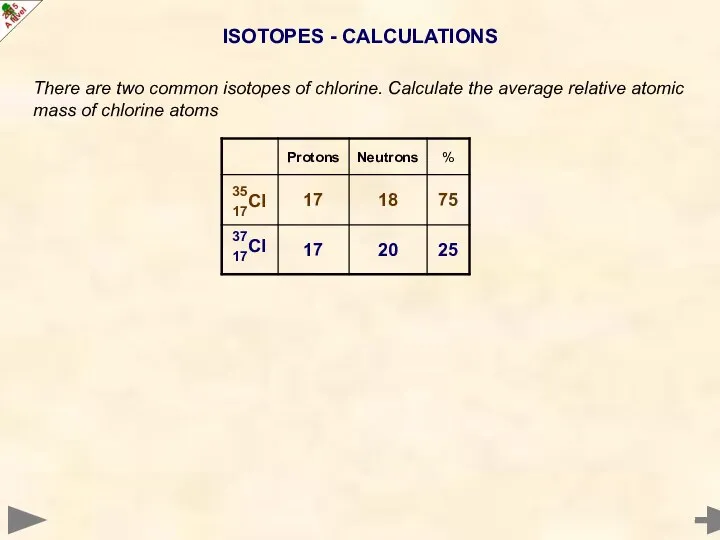
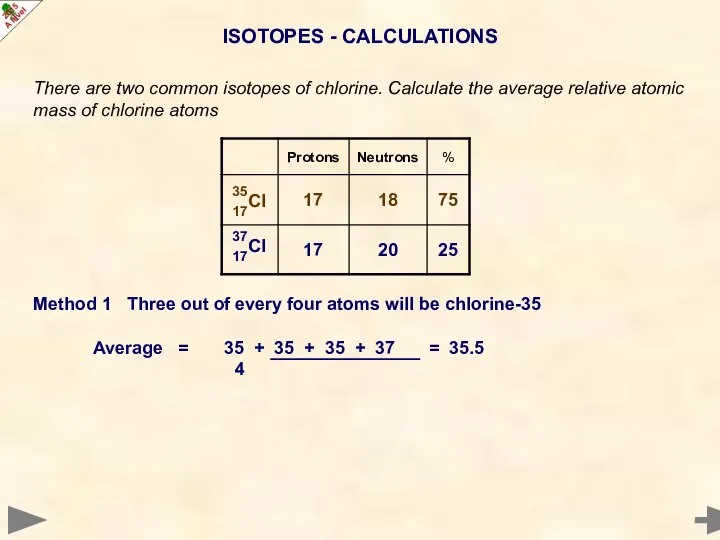
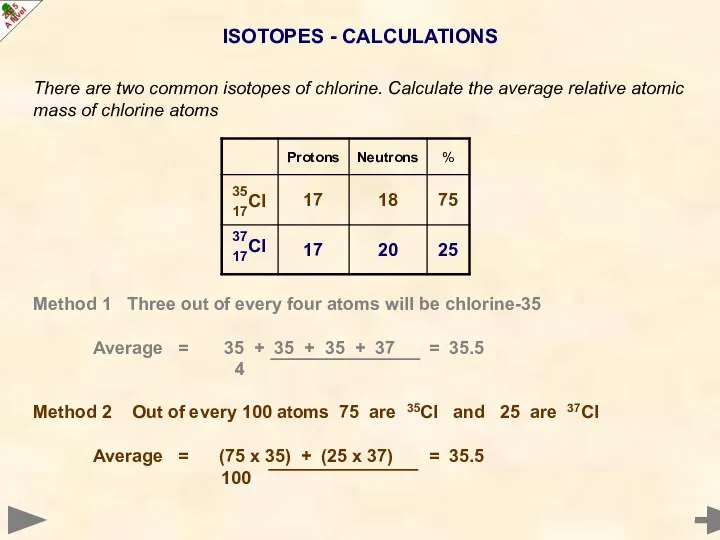
- 20. ISOTOPES - CALCULATIONS There are two common isotopes of chlorine. Calculate the average relative atomic mass
- 21. ISOTOPES - CALCULATIONS There are two common isotopes of chlorine. Calculate the average relative atomic mass
- 22. ISOTOPES - CALCULATIONS There are two common isotopes of chlorine. Calculate the average relative atomic mass
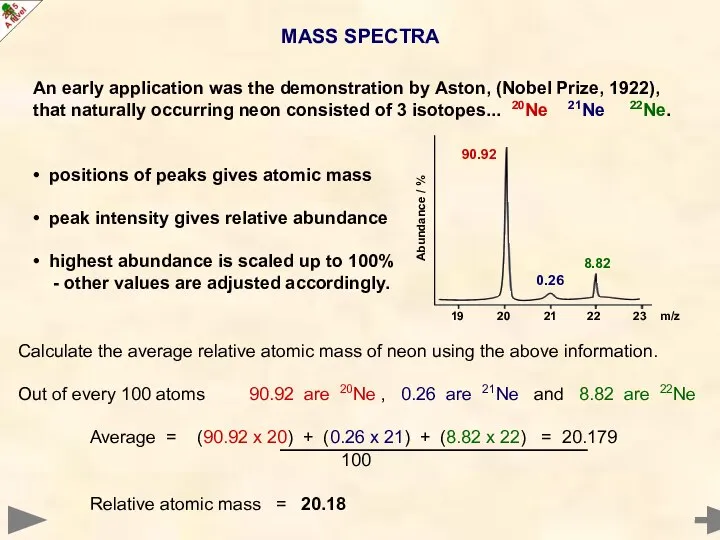
- 23. MASS SPECTRA An early application was the demonstration by Aston, (Nobel Prize, 1922), that naturally occurring
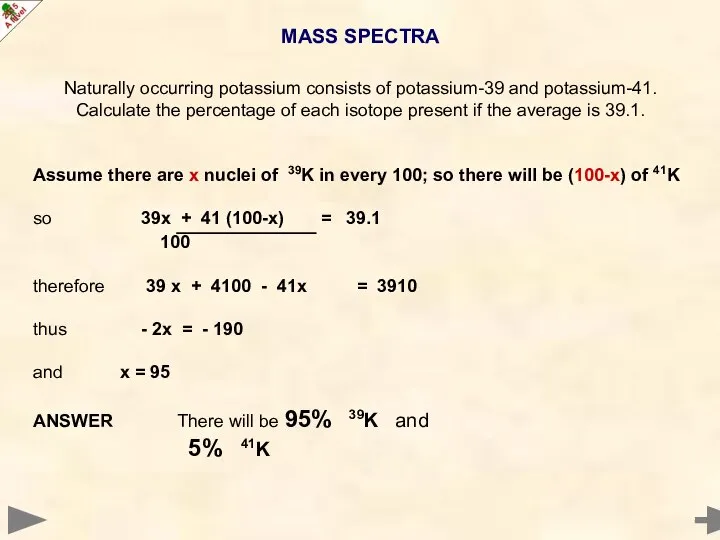
- 24. MASS SPECTRA Naturally occurring potassium consists of potassium-39 and potassium-41. Calculate the percentage of each isotope
- 26. Скачать презентацию



 Увлекательная химия
Увлекательная химия Термический анализ
Термический анализ Презентация по Химии "Презентация Медь" - скачать смотреть
Презентация по Химии "Презентация Медь" - скачать смотреть  Алканы и их свойства
Алканы и их свойства Класифікація розчинів. Осмос. Осмотичний тиск
Класифікація розчинів. Осмос. Осмотичний тиск Реакции ионного обмена в водных растворах электролитов. Ионные реакции и уравнения
Реакции ионного обмена в водных растворах электролитов. Ионные реакции и уравнения Основные положения теории электролитической диссоциации
Основные положения теории электролитической диссоциации Игра «Химические элементы» (формулы и названия)
Игра «Химические элементы» (формулы и названия) Биохимия и молекулярная биология. (Лекция 1)
Биохимия и молекулярная биология. (Лекция 1) Камни и Рыбы
Камни и Рыбы Минералы
Минералы Растворы электролитов
Растворы электролитов Материаловедение. Диаграммы состояния
Материаловедение. Диаграммы состояния Кремнийорганические модификаторы
Кремнийорганические модификаторы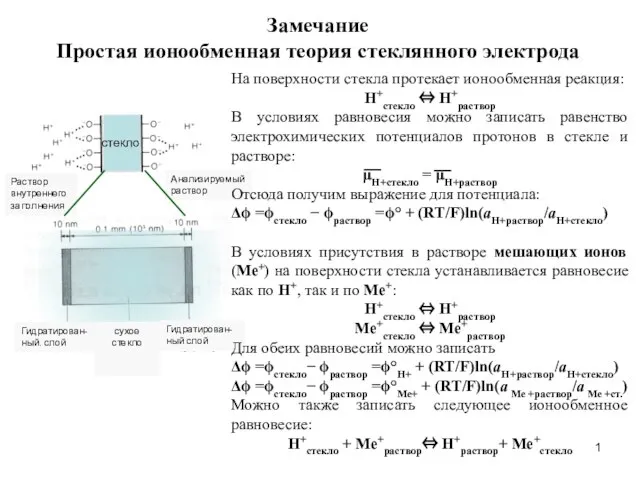 Простая ионообменная теория стеклянного электрода
Простая ионообменная теория стеклянного электрода Порцеляна. Фаянс
Порцеляна. Фаянс Физические свойства металлов
Физические свойства металлов Подгруппа углерода
Подгруппа углерода Направление окислительно-восстановительного процесса
Направление окислительно-восстановительного процесса Липиды - жиры и жироподобные органические соединения, практически нерастворимые в воде
Липиды - жиры и жироподобные органические соединения, практически нерастворимые в воде Минералогический и химический состав почвы
Минералогический и химический состав почвы Дисперсные системы и растворы
Дисперсные системы и растворы Неорганические и органические основания.
Неорганические и органические основания. Подготовка к ГИА. В1. Периодический закон Д.И. Менделеева
Подготовка к ГИА. В1. Периодический закон Д.И. Менделеева The role of chemistry in the solution of the food problem
The role of chemistry in the solution of the food problem Презентация Типы изомерий 10 класс
Презентация Типы изомерий 10 класс Загадочная медь
Загадочная медь Углерод. Кремний
Углерод. Кремний