Содержание
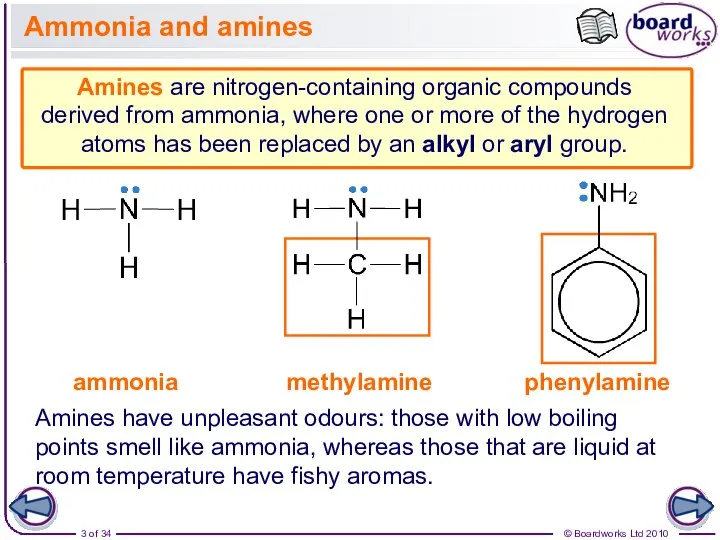
- 3. Ammonia and amines Amines are nitrogen-containing organic compounds derived from ammonia, where one or more of
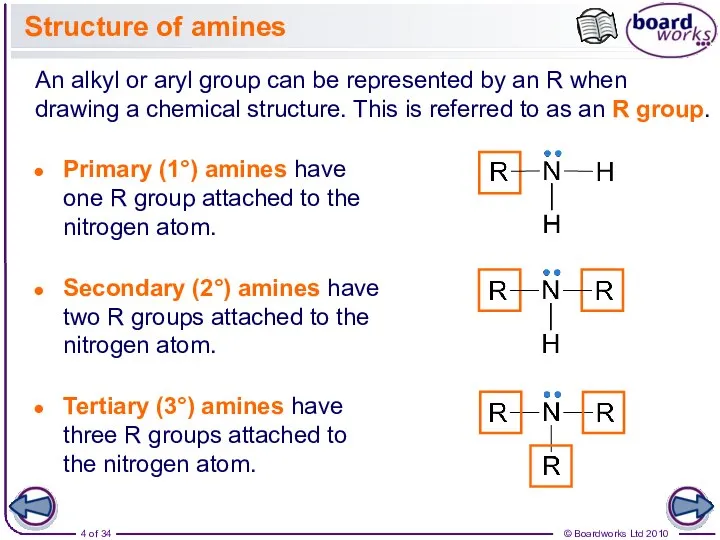
- 4. Structure of amines An alkyl or aryl group can be represented by an R when drawing
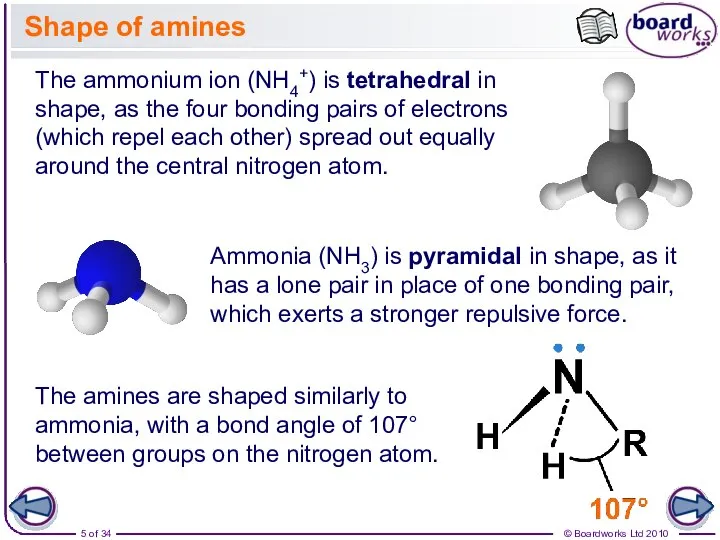
- 5. Shape of amines The ammonium ion (NH4+) is tetrahedral in shape, as the four bonding pairs
- 6. Identifying amines
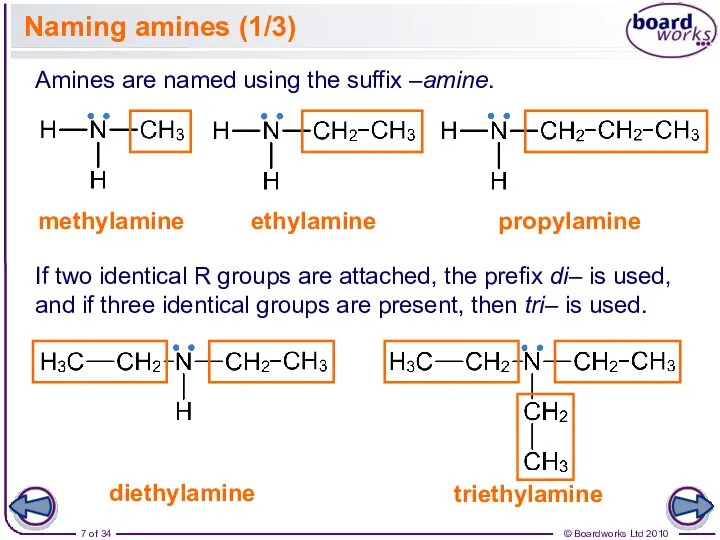
- 7. Naming amines (1/3) Amines are named using the suffix –amine. methylamine ethylamine propylamine diethylamine If two
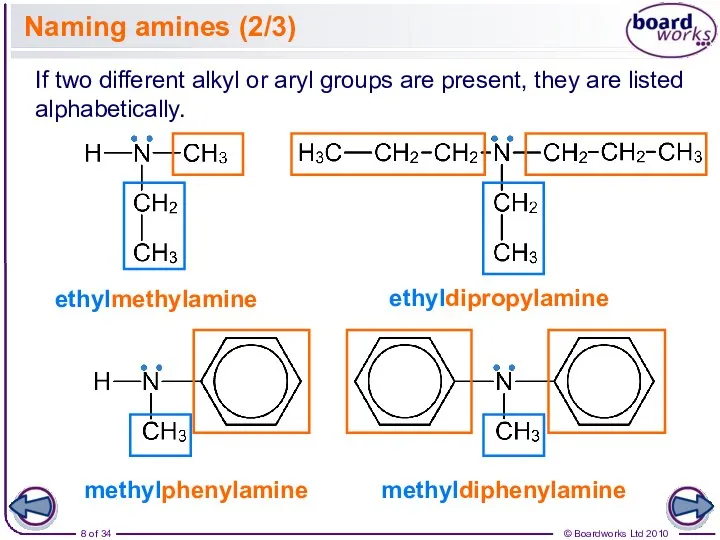
- 8. Naming amines (2/3) ethylmethylamine If two different alkyl or aryl groups are present, they are listed
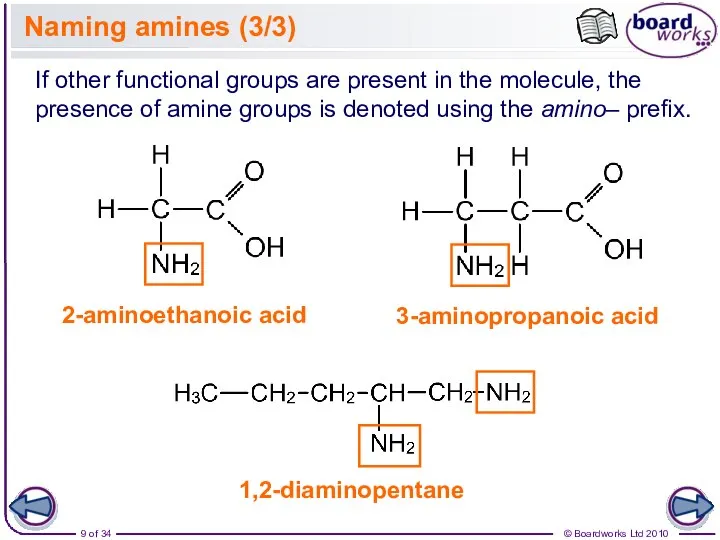
- 9. Naming amines (3/3) 3-aminopropanoic acid If other functional groups are present in the molecule, the presence
- 10. Naming amines activity
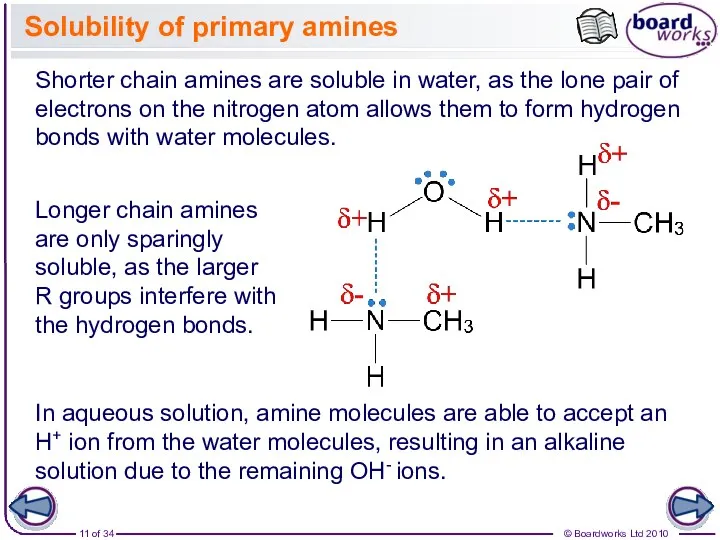
- 11. Solubility of primary amines Longer chain amines are only sparingly soluble, as the larger R groups
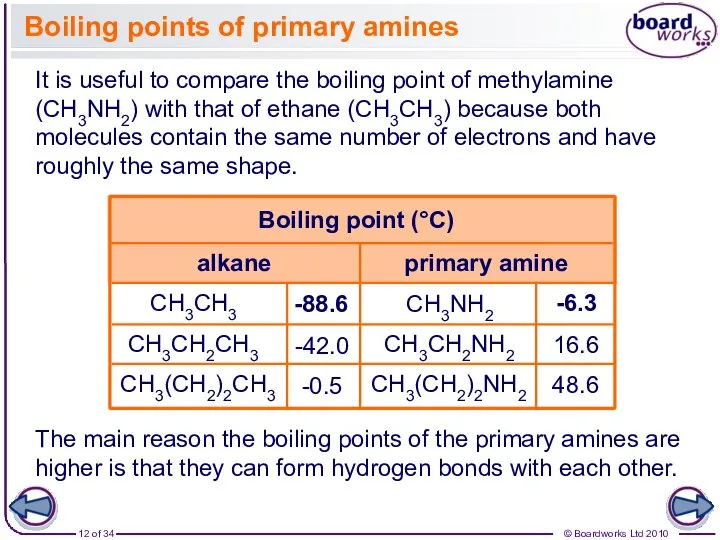
- 12. CH3NH2 CH3CH2NH2 CH3(CH2)2NH2 Boiling points of primary amines It is useful to compare the boiling point
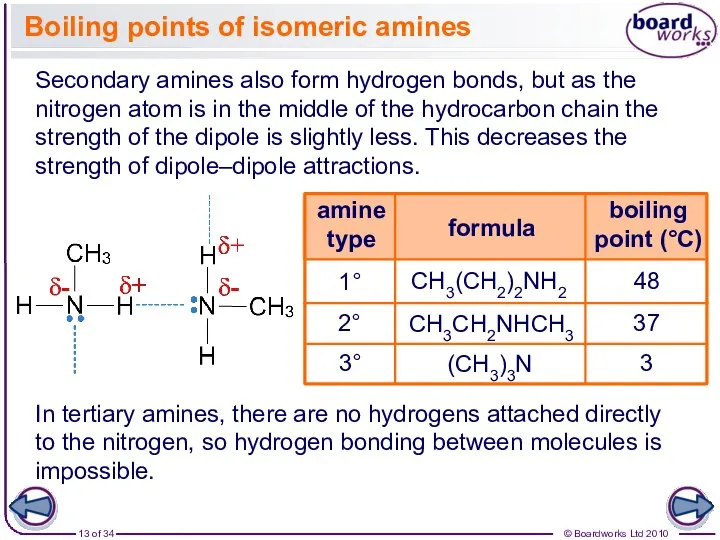
- 13. In tertiary amines, there are no hydrogens attached directly to the nitrogen, so hydrogen bonding between
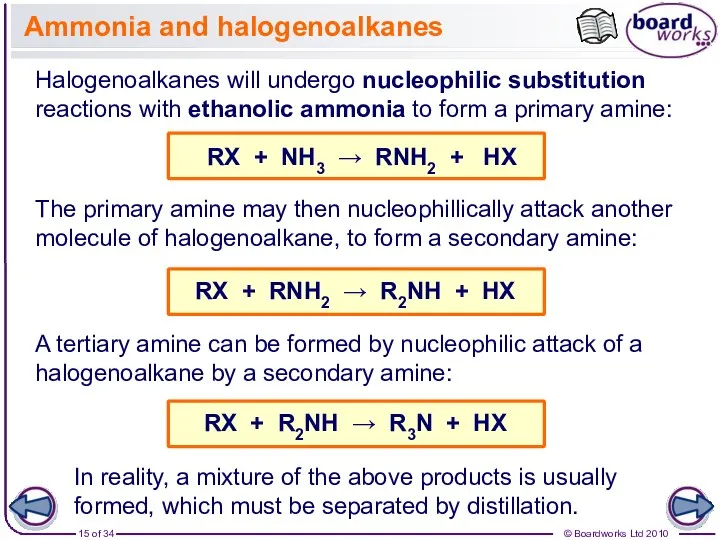
- 15. Ammonia and halogenoalkanes Halogenoalkanes will undergo nucleophilic substitution reactions with ethanolic ammonia to form a primary
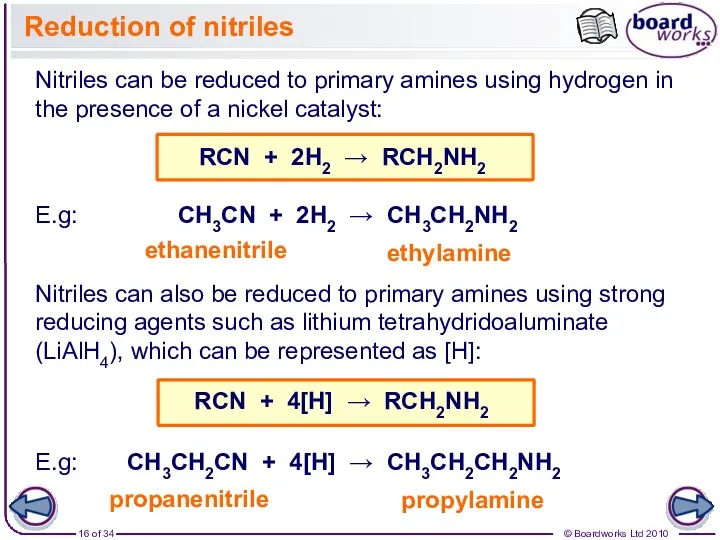
- 16. Reduction of nitriles Nitriles can be reduced to primary amines using hydrogen in the presence of
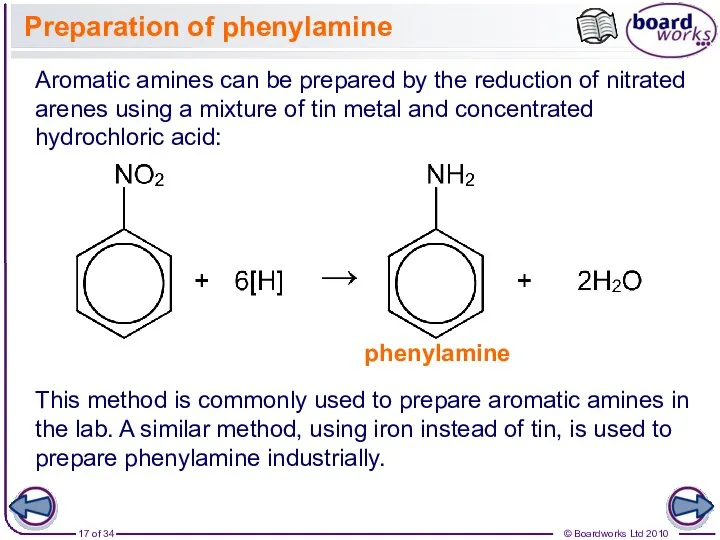
- 17. Preparation of phenylamine Aromatic amines can be prepared by the reduction of nitrated arenes using a
- 18. Which conditions?
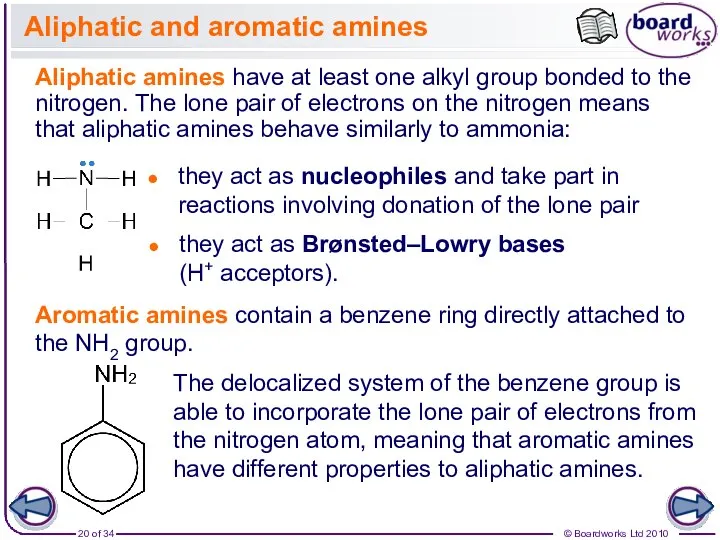
- 20. Aliphatic and aromatic amines Aliphatic amines have at least one alkyl group bonded to the nitrogen.
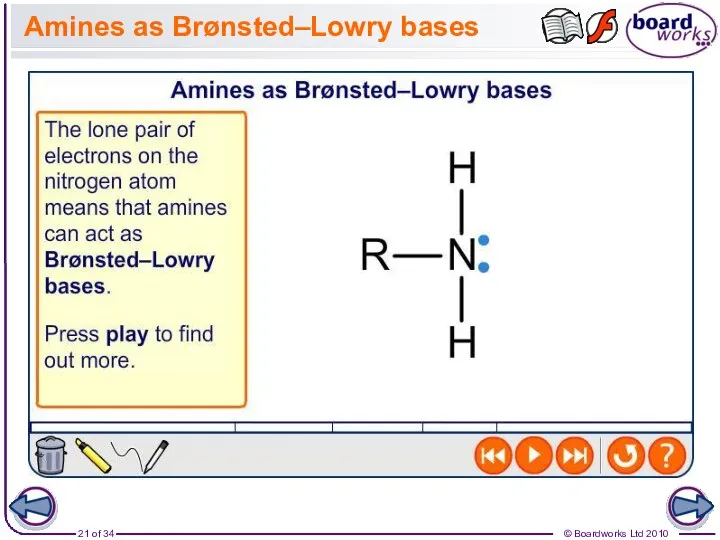
- 21. Amines as Brønsted–Lowry bases
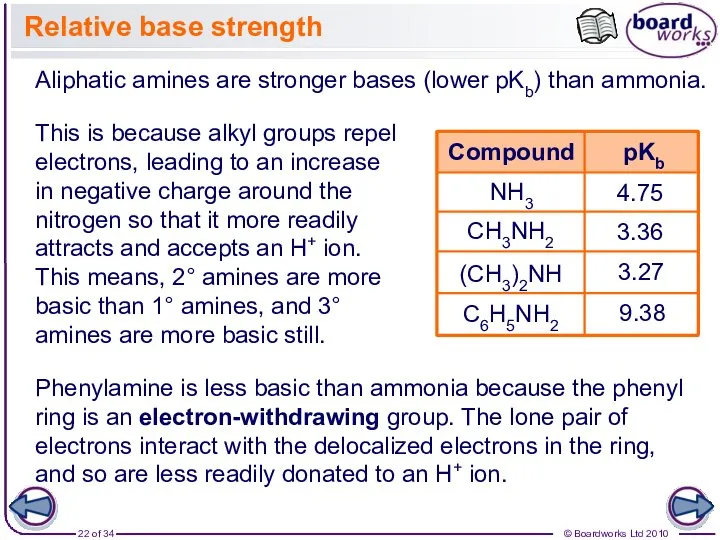
- 22. Relative base strength NH3 4.75 3.36 3.27 Phenylamine is less basic than ammonia because the phenyl
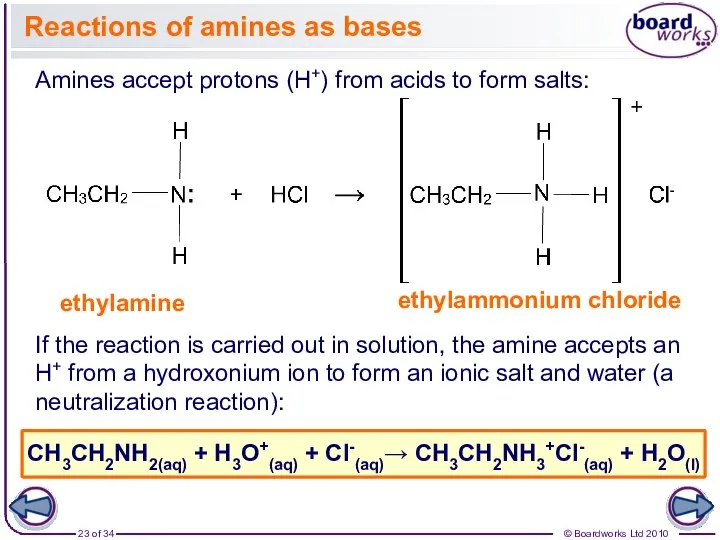
- 23. Reactions of amines as bases Amines accept protons (H+) from acids to form salts: ethylamine ethylammonium
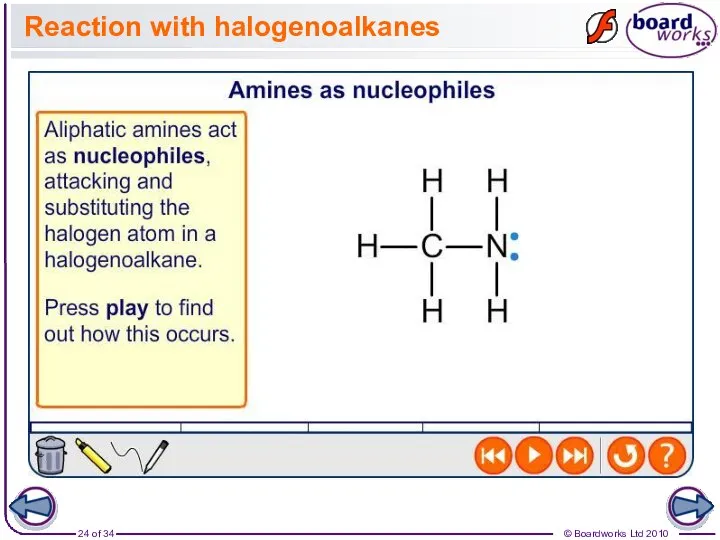
- 24. Reaction with halogenoalkanes
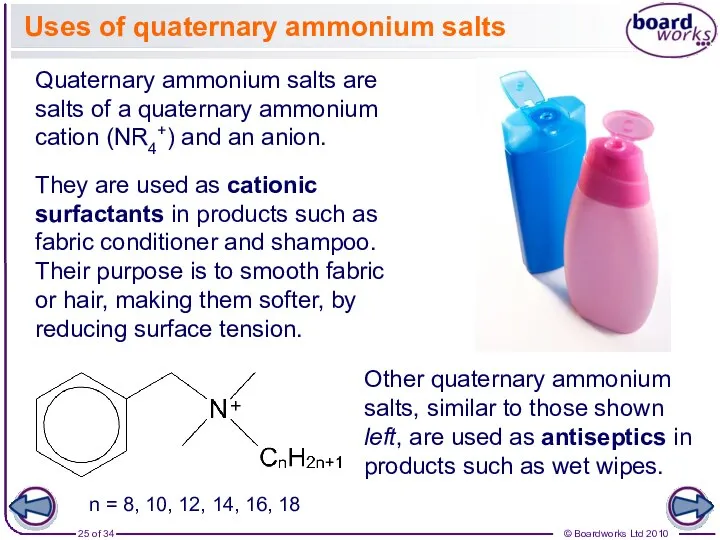
- 25. Uses of quaternary ammonium salts Quaternary ammonium salts are salts of a quaternary ammonium cation (NR4+)
- 26. Reaction with acyl compounds
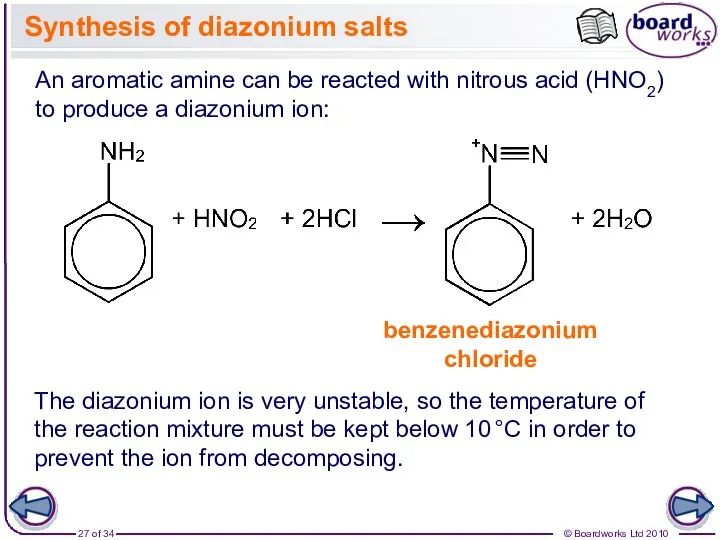
- 27. Synthesis of diazonium salts An aromatic amine can be reacted with nitrous acid (HNO2) to produce
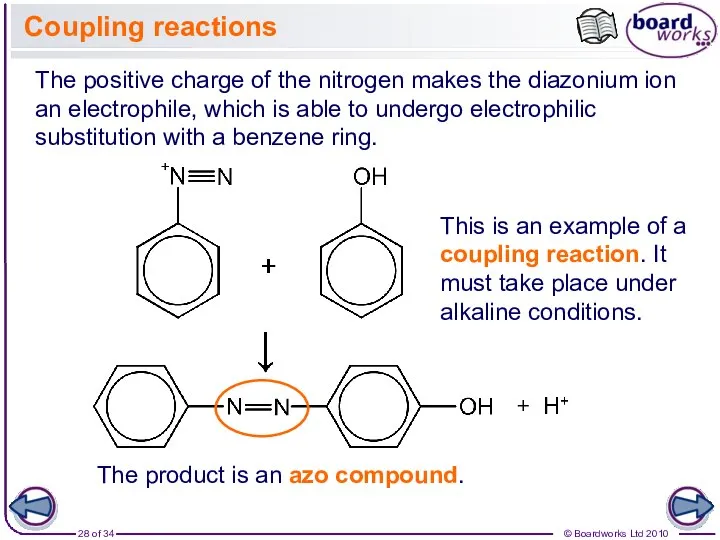
- 28. Coupling reactions The positive charge of the nitrogen makes the diazonium ion an electrophile, which is
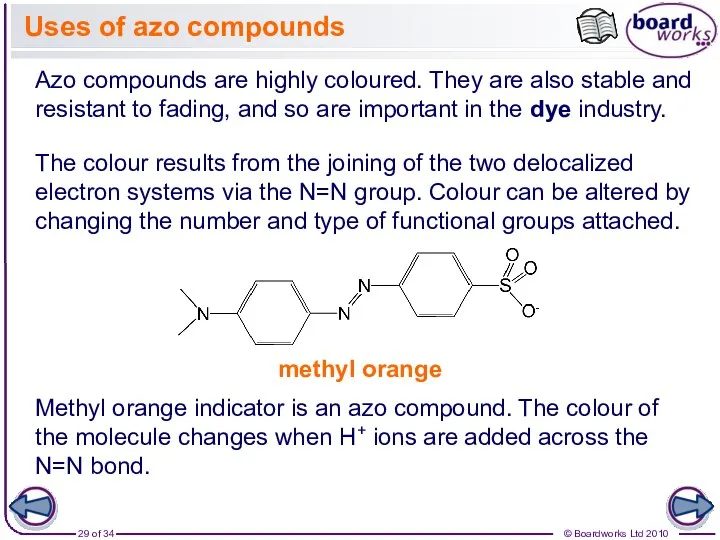
- 29. Uses of azo compounds Azo compounds are highly coloured. They are also stable and resistant to
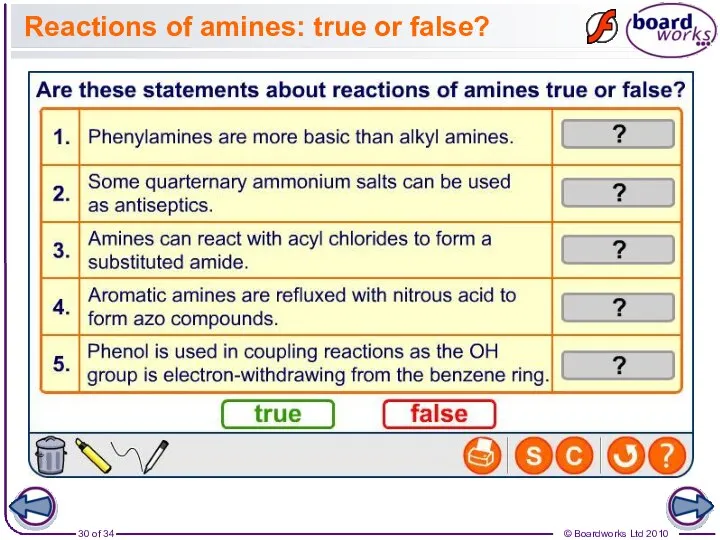
- 30. Reactions of amines: true or false?
- 32. Glossary
- 33. What’s the keyword?
- 35. Скачать презентацию



 Области применения серной, соляной, азотной, и уксусной кислот
Области применения серной, соляной, азотной, и уксусной кислот Углерод. Металлы. 9 класс
Углерод. Металлы. 9 класс Алюминий и его соединения
Алюминий и его соединения Біогеохімічні цикли та їх еволюція
Біогеохімічні цикли та їх еволюція Презентация Витамин Е
Презентация Витамин Е  Фазовые диаграммы бинарных систем с полиморфными превращениями на примере фазовой диаграммы системы железо-цементит Fe Fe3C
Фазовые диаграммы бинарных систем с полиморфными превращениями на примере фазовой диаграммы системы железо-цементит Fe Fe3C Естествознание. Раздел II. Химия с элементами экологии Урок по теме:
Естествознание. Раздел II. Химия с элементами экологии Урок по теме: Презентация по Химии "Презентация Войди в природу другом" - скачать смотреть
Презентация по Химии "Презентация Войди в природу другом" - скачать смотреть  Презентация по Химии "Пластмаса" - скачать смотреть бесплатно
Презентация по Химии "Пластмаса" - скачать смотреть бесплатно Кислотно-основное титрование. 4 лекция. Часть 2
Кислотно-основное титрование. 4 лекция. Часть 2 Реакции координированных лигандов
Реакции координированных лигандов Экспертные системы распознавания химических веществ
Экспертные системы распознавания химических веществ Презентация по Химии "Глюкоза" - скачать смотреть
Презентация по Химии "Глюкоза" - скачать смотреть  Общие представления об электрохимических технологиях
Общие представления об электрохимических технологиях Основные классы неорганических соединений
Основные классы неорганических соединений Электролиз
Электролиз ГОД ЭКОЛОГИИ-2013
ГОД ЭКОЛОГИИ-2013  Растворы
Растворы Что объединяет вещества. Классификация твёрдых веществ
Что объединяет вещества. Классификация твёрдых веществ Химия и здоровье
Химия и здоровье  Элементный, фракционный и химический состав нефти. Классификация нефтей
Элементный, фракционный и химический состав нефти. Классификация нефтей В чём вкус хлеба?
В чём вкус хлеба? Катализ.Лекция
Катализ.Лекция Антибиотики как ЛС
Антибиотики как ЛС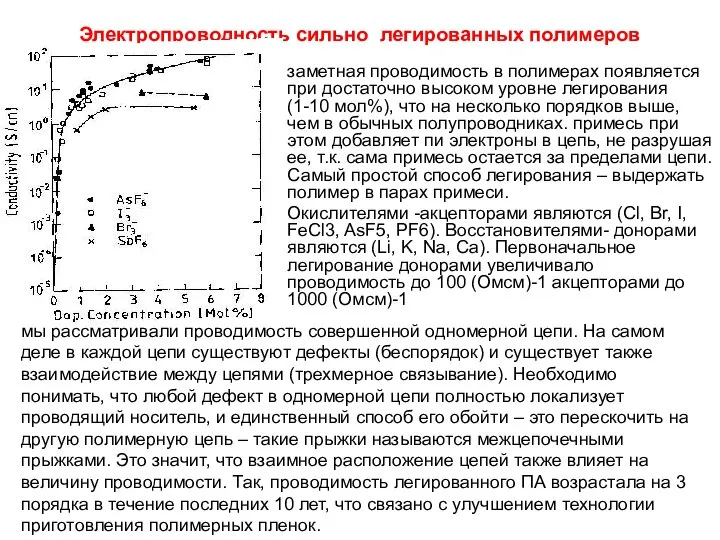 Электропроводность сильно легированных полимеров заметная проводимость в полимерах появляется при достаточно высоком уров
Электропроводность сильно легированных полимеров заметная проводимость в полимерах появляется при достаточно высоком уров КВН В МИРЕ ВЕЩЕСТВ
КВН В МИРЕ ВЕЩЕСТВ Полимерные реагенты в бурении
Полимерные реагенты в бурении Хімічний лабіринт
Хімічний лабіринт