Содержание
- 2. Definition of ceramics Metals - consist of atoms held together by delocalized electrons - overcome the
- 3. Definition of ceramics Metals - Alloys : combinations of metallic elements or metallic and nonmetallic elements
- 4. Definition of ceramics Metals - SC (Simple Cubic Structure) Coordination # = 6 Atomic Packing Factor
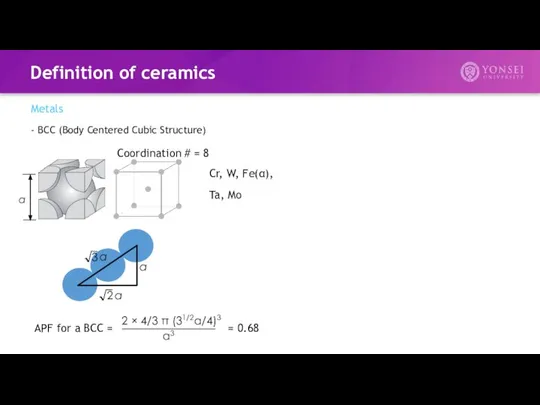
- 5. Definition of ceramics Metals - BCC (Body Centered Cubic Structure) APF for a BCC = =
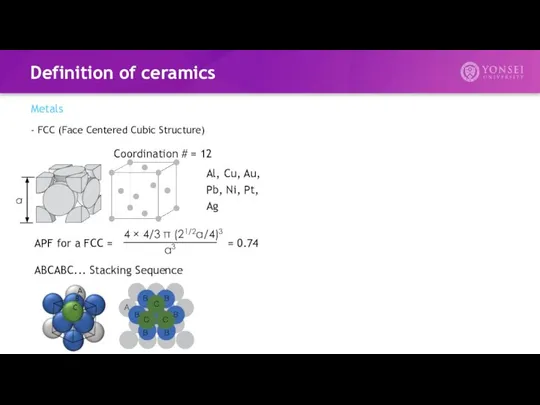
- 6. Definition of ceramics Metals - FCC (Face Centered Cubic Structure) APF for a FCC = =
- 7. Definition of ceramics Metals - HCP (Hexagonal Close Packed Structure) Coordination # = 12 Cd, Mg,
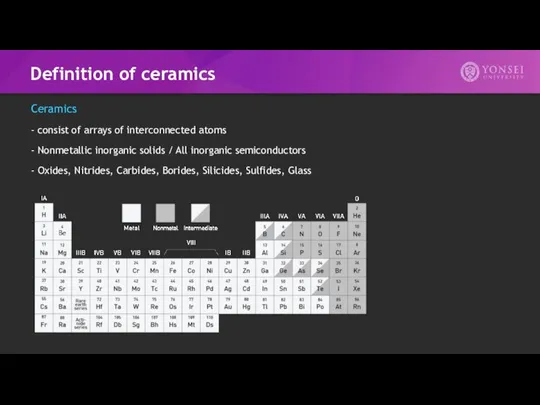
- 8. Definition of ceramics Ceramics - consist of arrays of interconnected atoms - Nonmetallic inorganic solids /
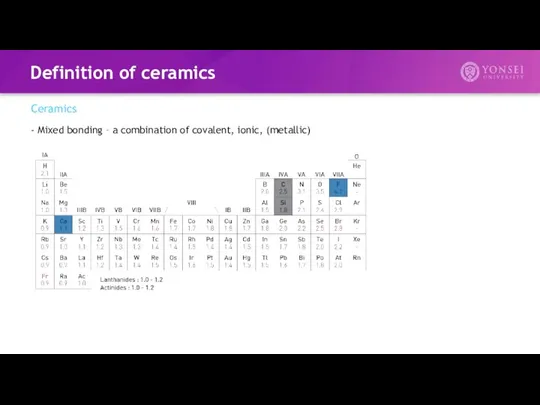
- 9. Definition of ceramics Ceramics - Mixed bonding – a combination of covalent, ionic, (metallic)
- 10. Definition of ceramics Ceramics - Crystal structures – Oxide structures ⋅ Ionic radius of oxygen anion
- 11. Definition of ceramics Ceramics Charge neutrality : Net charge = 0 ⋅ Composition : Ma+xAb-y ?
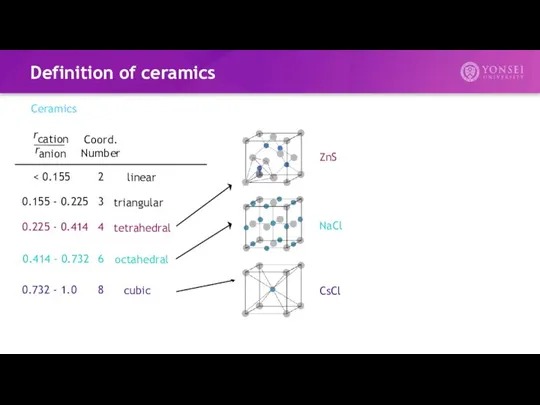
- 12. Definition of ceramics Ceramics 2 Coord. Number 0.155 - 0.225 0.225 - 0.414 0.414 - 0.732
- 14. Скачать презентацию






 Водород в космосе
Водород в космосе Структурная организация биополимеров. ДНК и РНК
Структурная организация биополимеров. ДНК и РНК Презентация по Химии "Кто хочет стать отличником" - скачать смотреть
Презентация по Химии "Кто хочет стать отличником" - скачать смотреть  Электрохимические процессы
Электрохимические процессы Витамины и авитаминоз - презентация_
Витамины и авитаминоз - презентация_ Презентация по Химии "Пероксид водорода" - скачать смотреть
Презентация по Химии "Пероксид водорода" - скачать смотреть  Кислоты. (8 класс.)
Кислоты. (8 класс.) Теоретическая электрохимия, часть 1
Теоретическая электрохимия, часть 1 Композиционные материалы
Композиционные материалы Технология адсорбционной осушки газа
Технология адсорбционной осушки газа Русские ученые-химики
Русские ученые-химики Окислительно-восстановительные реакции
Окислительно-восстановительные реакции Лекарственное растительное сырье, содержащее производные антрацена
Лекарственное растительное сырье, содержащее производные антрацена Органические вещества
Органические вещества Основания как электролиты
Основания как электролиты 8 класс
8 класс  Алканы. Свойства. Строение и применение
Алканы. Свойства. Строение и применение Неорганические вещества
Неорганические вещества Углеводы. Общая характеристика углеводов
Углеводы. Общая характеристика углеводов Kristālisku vielu uzbūve
Kristālisku vielu uzbūve Симметрия в химии. Кристаллы
Симметрия в химии. Кристаллы Аттестационная работа. Образовательная программа элективного курса по химии
Аттестационная работа. Образовательная программа элективного курса по химии Минерал клинохлор. Месторождения
Минерал клинохлор. Месторождения Полимеры. Пластмассы. Волокна. Цели: - узнать что такое пластмассы, волокна их отличие от полимеров; - изучить классификацию пл
Полимеры. Пластмассы. Волокна. Цели: - узнать что такое пластмассы, волокна их отличие от полимеров; - изучить классификацию пл Липиды - жиры и жироподобные органические соединения, практически нерастворимые в воде
Липиды - жиры и жироподобные органические соединения, практически нерастворимые в воде Металлы; их классификация, строения и свойства
Металлы; их классификация, строения и свойства Геолого-промышленные типы месторождений полезных ископаемых
Геолого-промышленные типы месторождений полезных ископаемых Количество вещества
Количество вещества