Содержание
- 2. General aspects of chemical structure and reactivity of organic compounds Lecture №1
- 3. Chemical bonding and mutual atoms’ influence in organic molecules
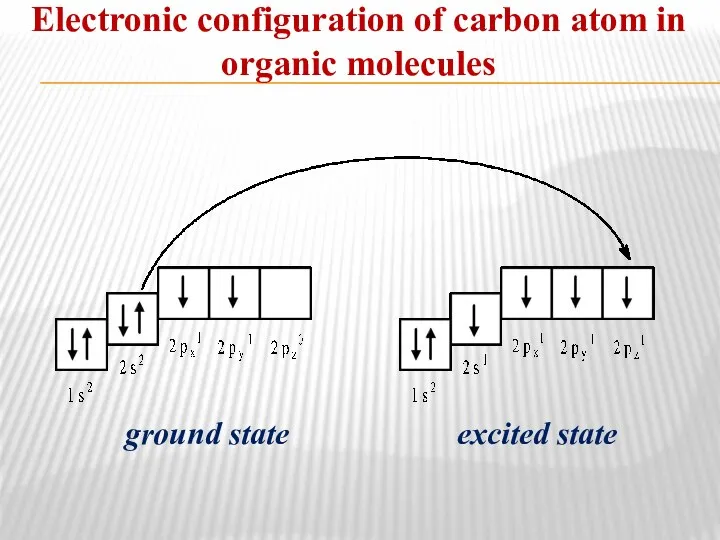
- 4. Electronic configuration of carbon atom in organic molecules ground state excited state
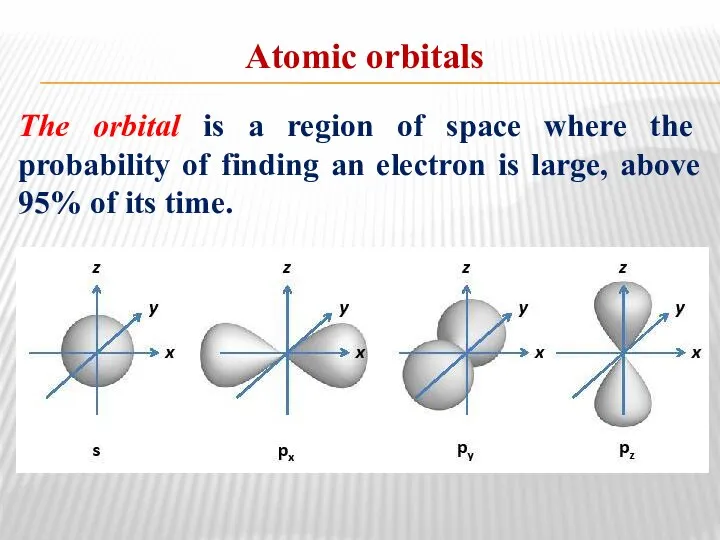
- 5. Atomic orbitals The orbital is a region of space where the probability of finding an electron
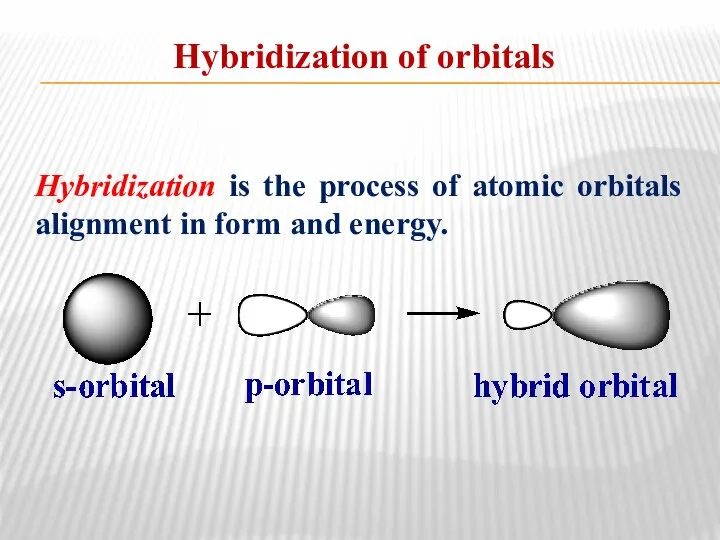
- 6. Hybridization of orbitals Hybridization is the process of atomic orbitals alignment in form and energy.
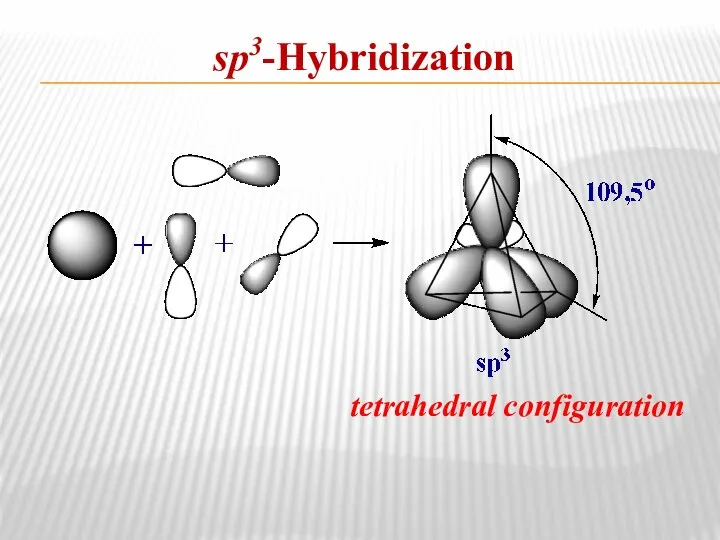
- 7. sp3-Hybridization tetrahedral configuration
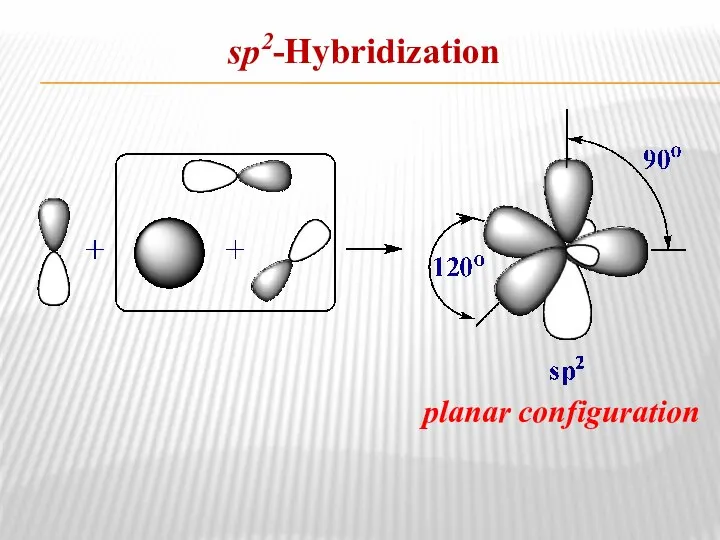
- 8. sp2-Hybridization planar configuration
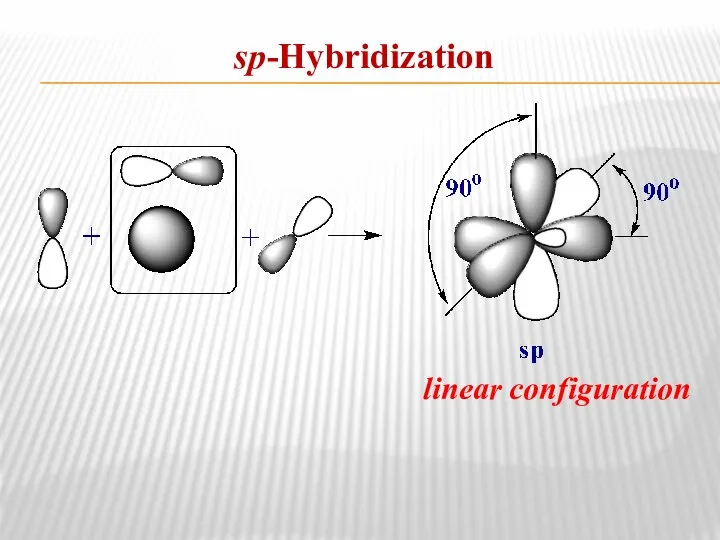
- 9. sp-Hybridization linear configuration
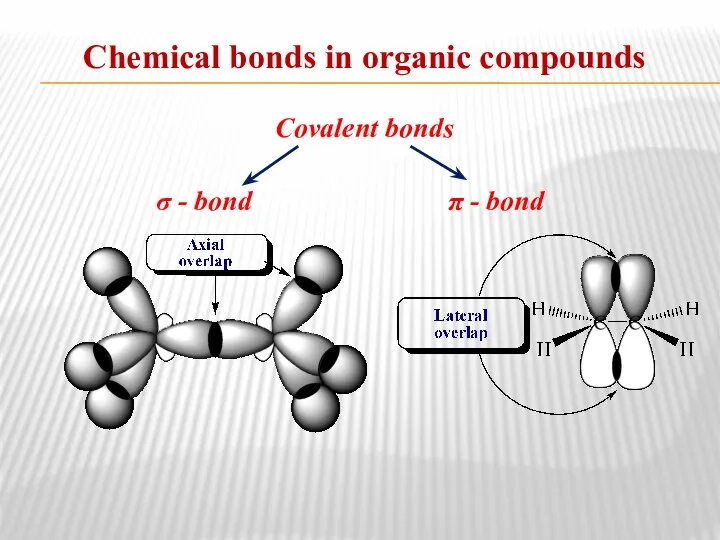
- 10. Chemical bonds in organic compounds Covalent bonds σ - bond π - bond
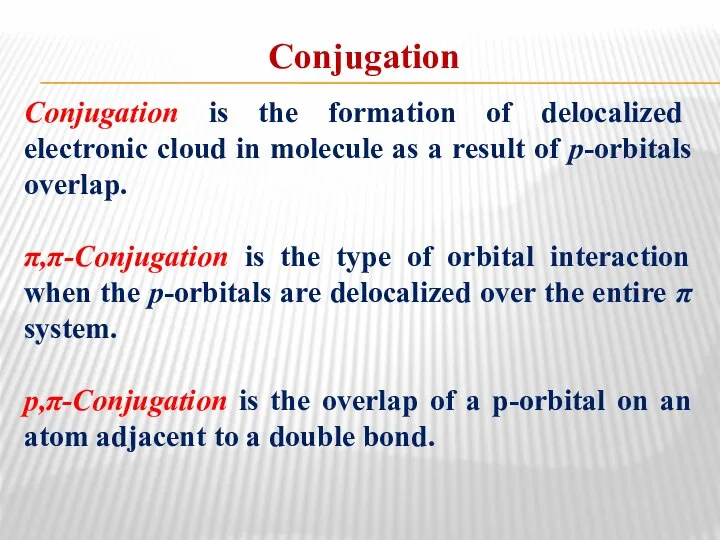
- 11. Conjugation Conjugation is the formation of delocalized electronic cloud in molecule as a result of p-orbitals
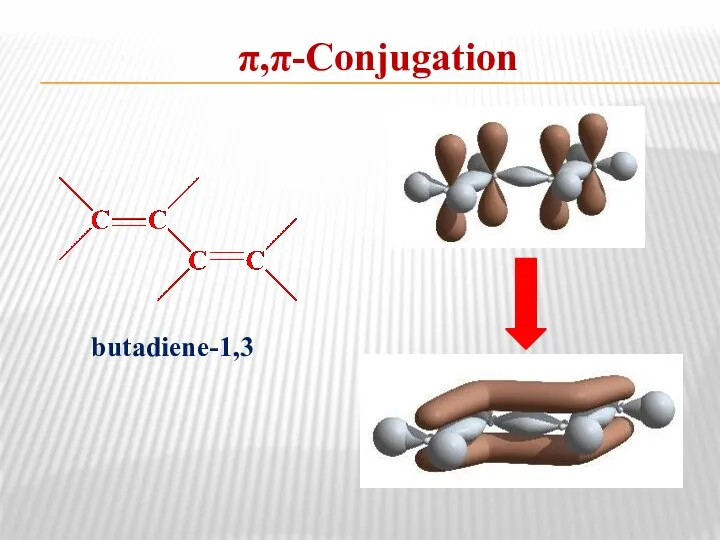
- 12. π,π-Conjugation butadiene-1,3
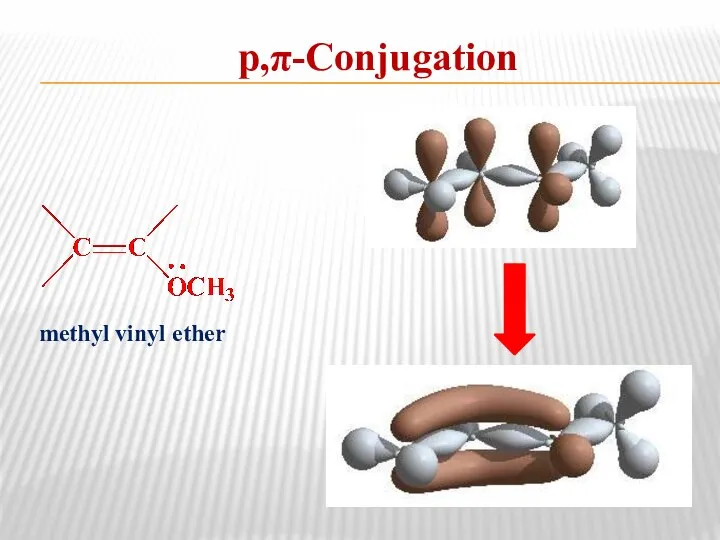
- 13. р,π-Conjugation methyl vinyl ether
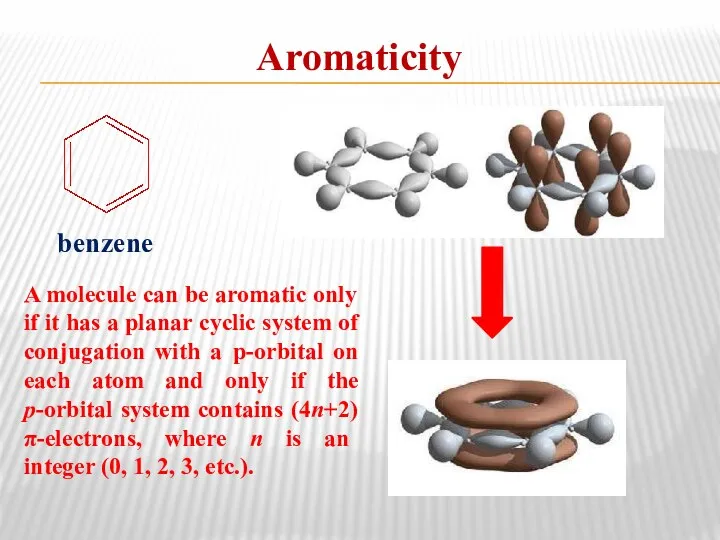
- 14. Aromaticity benzene A molecule can be aromatic only if it has a planar cyclic system of
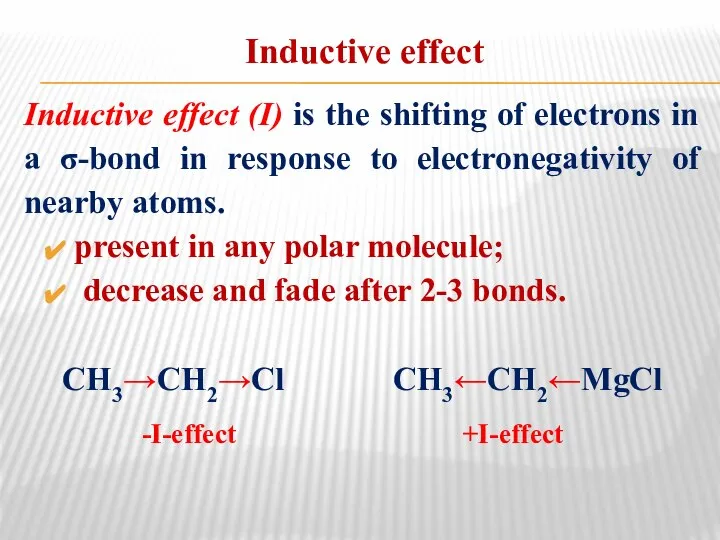
- 15. Inductive effect Inductive effect (I) is the shifting of electrons in a σ-bond in response to
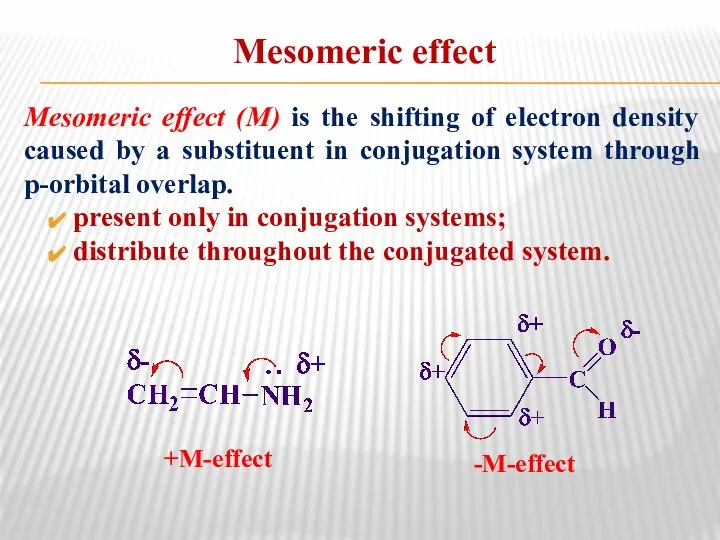
- 16. Mesomeric effect Mesomeric effect (М) is the shifting of electron density caused by a substituent in
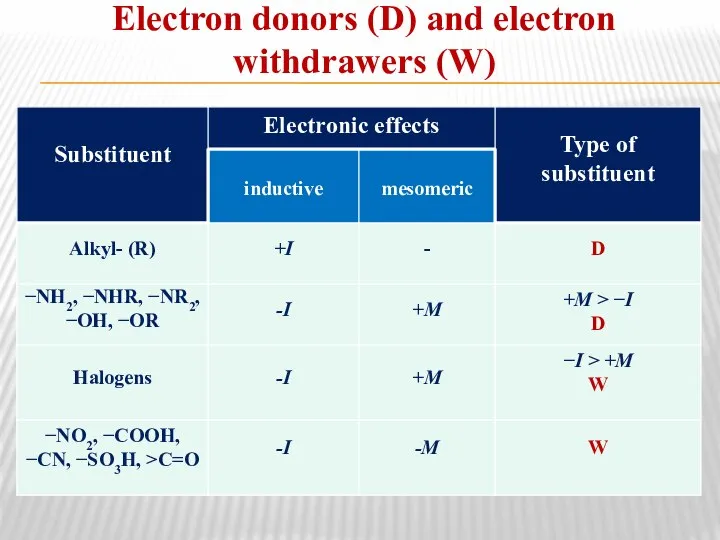
- 17. Electron donors (D) and electron withdrawers (W)
- 18. Spatial structure of organic compounds
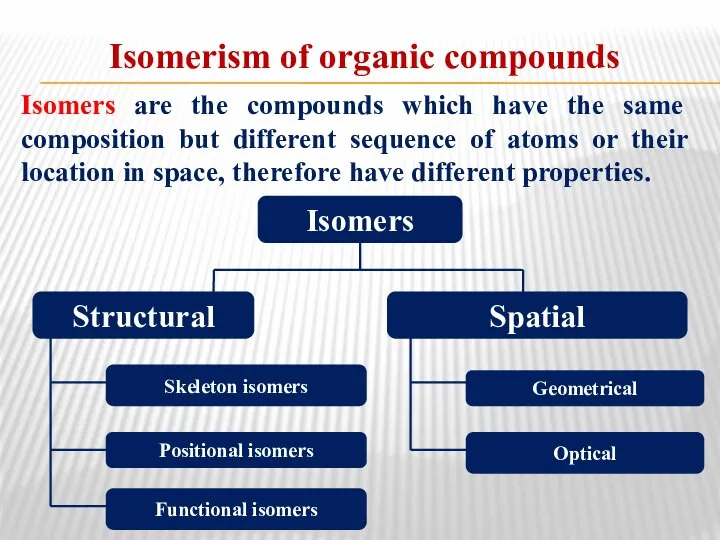
- 19. Isomerism of organic compounds Isomers are the compounds which have the same composition but different sequence
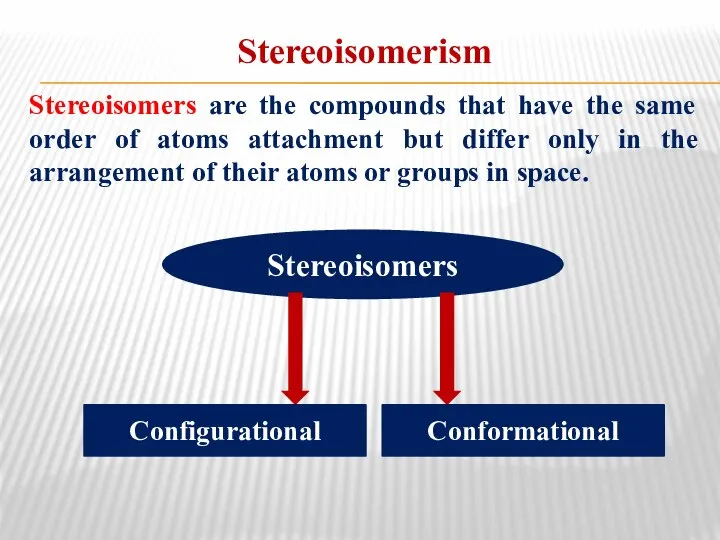
- 20. Stereoisomerism Stereoisomers are the compounds that have the same order of atoms attachment but differ only
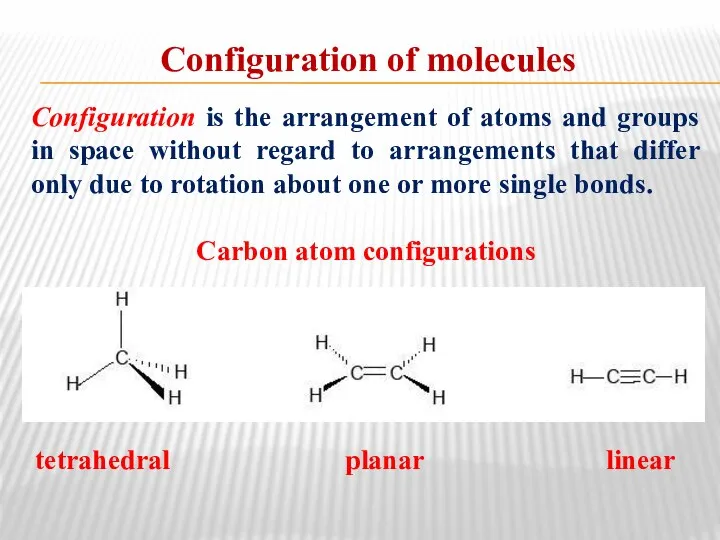
- 21. Configuration is the arrangement of atoms and groups in space without regard to arrangements that differ
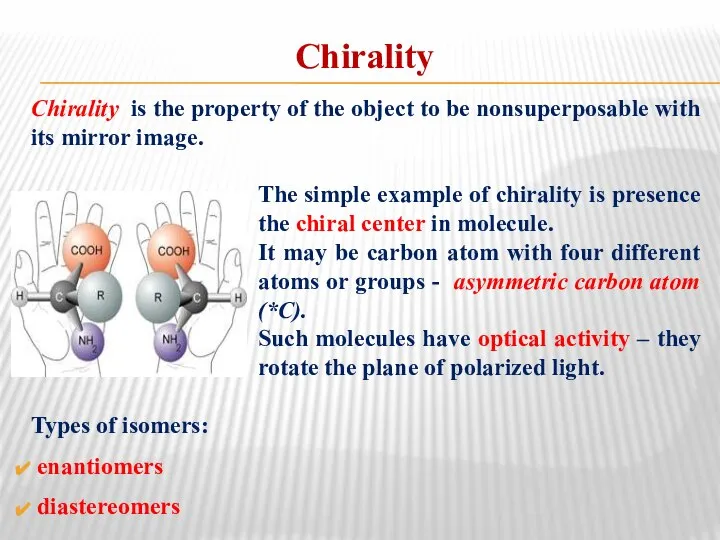
- 22. Chirality Chirality is the property of the object to be nonsuperposable with its mirror image. The
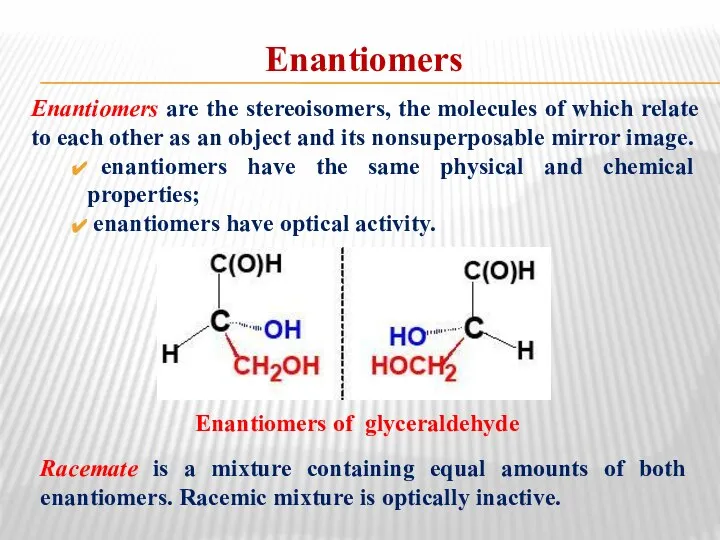
- 23. Enantiomers Enantiomers are the stereoisomers, the molecules of which relate to each other as an object
- 24. Fischer projections Spatial formulas Fischer projections Rules the carbon chain is disposed vertically (with the principle
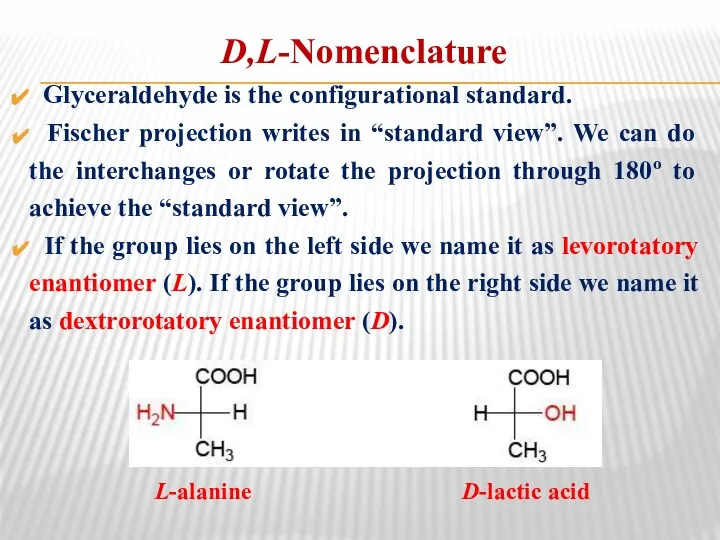
- 25. D,L-Nomenclature Glyceraldehyde is the configurational standard. Fischer projection writes in “standard view”. We can do the
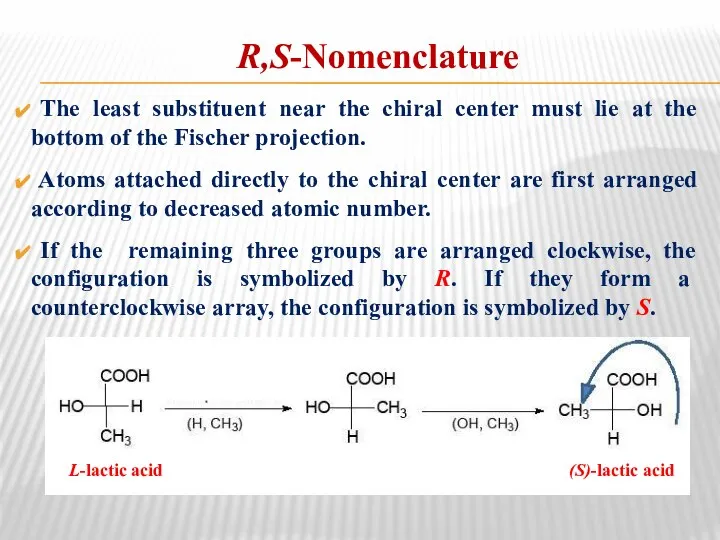
- 26. R,S-Nomenclature The least substituent near the chiral center must lie at the bottom of the Fischer
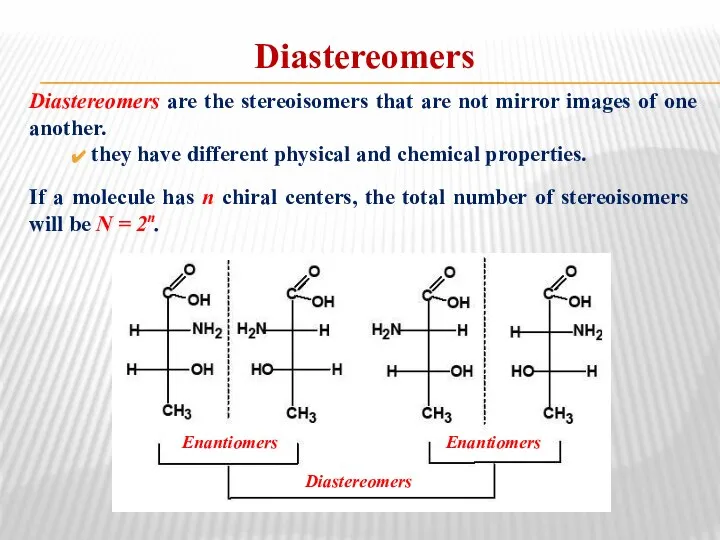
- 27. Diastereomers Diastereomers are the stereoisomers that are not mirror images of one another. they have different
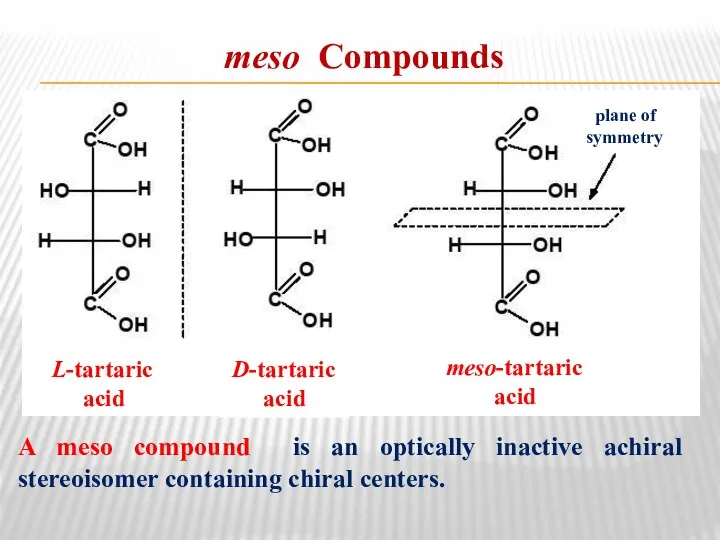
- 28. meso Compounds A meso compound is an optically inactive achiral stereoisomer containing chiral centers. L-tartaric acid
- 29. Acidity and basicity of organic compounds
- 30. Acidity and basicity are the key notions, determining many fundamental physico-chemical and biochemical properties of organic
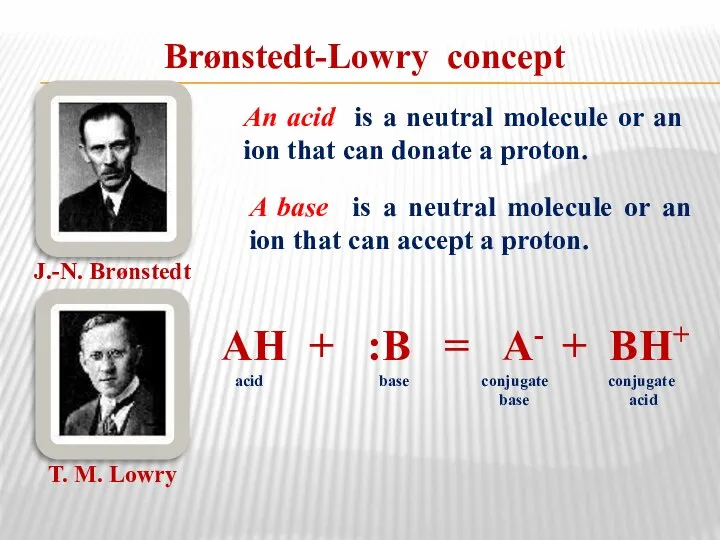
- 31. Brønstedt-Lowry concept J.-N. Brønstedt Т. М. Lowry An acid is a neutral molecule or an ion
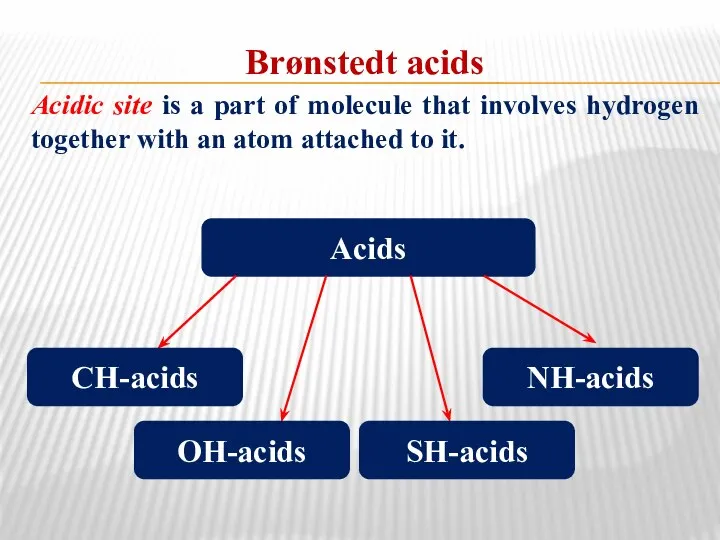
- 32. Brønstedt acids Acidic site is a part of molecule that involves hydrogen together with an atom
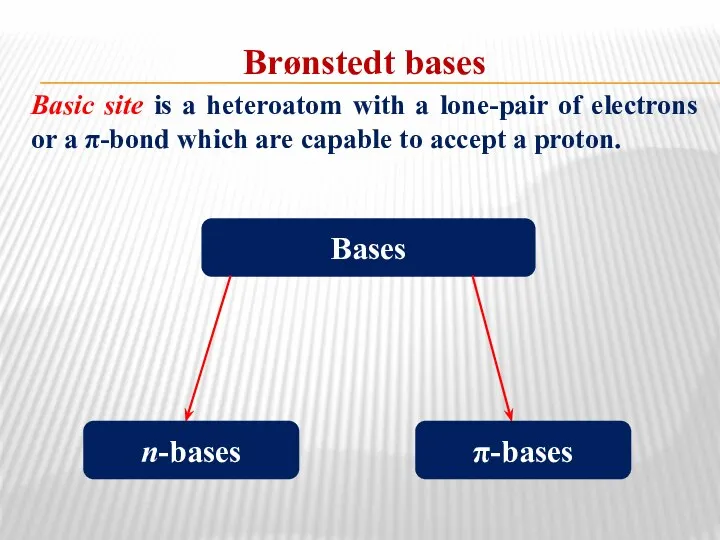
- 33. Brønstedt bases Basic site is a heteroatom with a lone-pair of electrons or a π-bond which
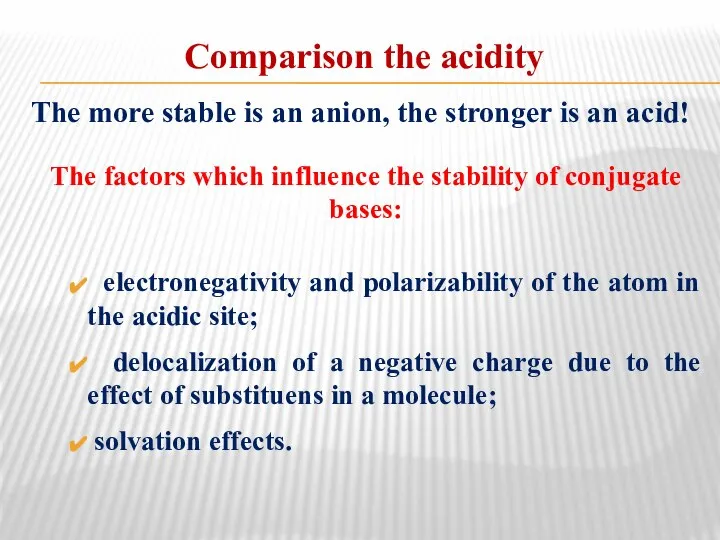
- 34. Comparison the acidity The more stable is an anion, the stronger is an acid! The factors
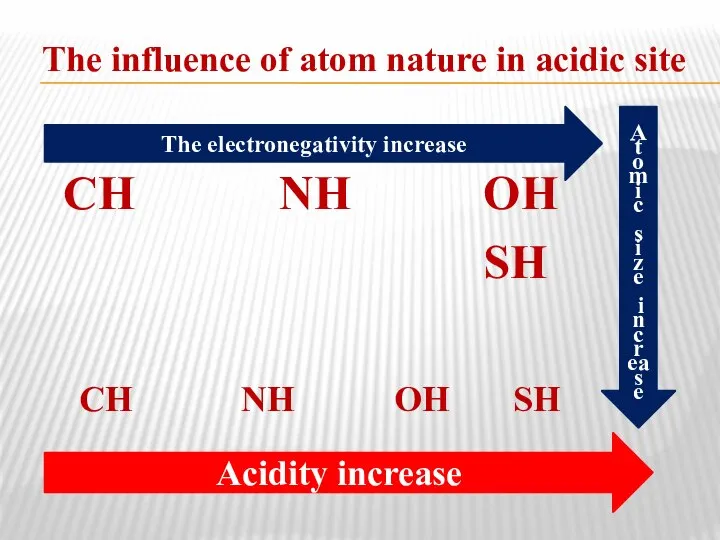
- 35. The influence of atom nature in acidic site СН NH ОН SH СН NH ОН SH
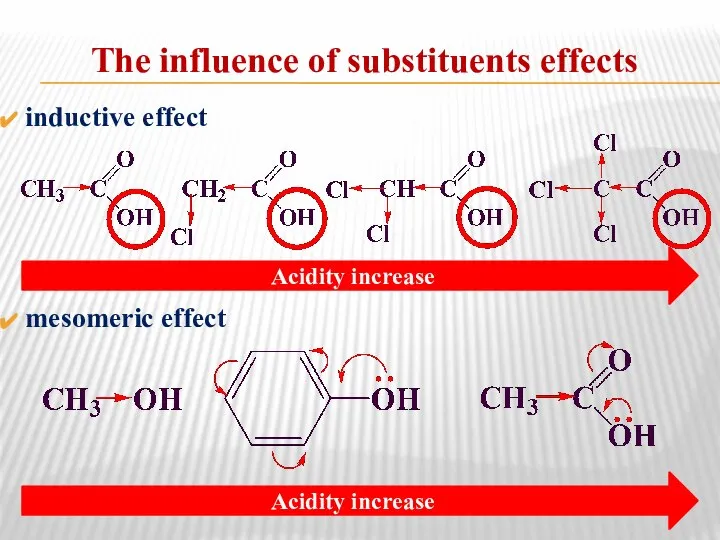
- 36. The influence of substituents effects inductive effect mesomeric effect Acidity increase Acidity increase
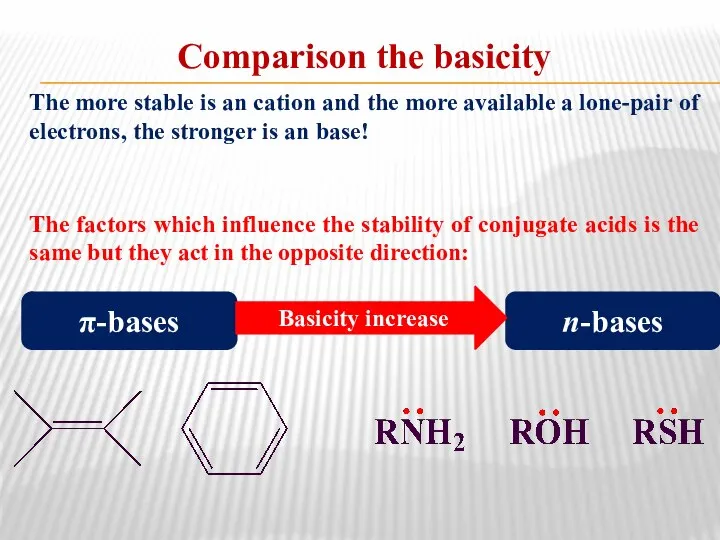
- 37. Comparison the basicity The more stable is an cation and the more available a lone-pair of
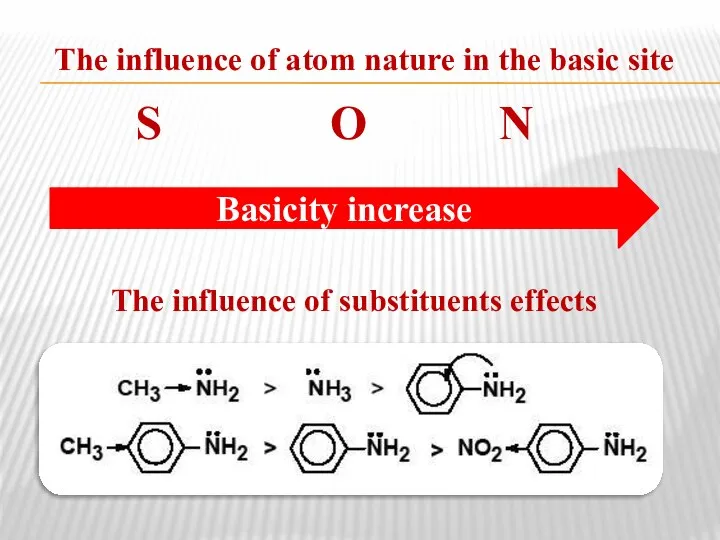
- 38. The influence of atom nature in the basic site S О N The influence of substituents
- 40. Скачать презентацию



 Общая и неорганическая химия
Общая и неорганическая химия Стратегії дослідження хімічних сполук з використанням сучасних фізичних методів (частина друга)
Стратегії дослідження хімічних сполук з використанням сучасних фізичних методів (частина друга) Високомолекулярні сполуки
Високомолекулярні сполуки Химические свойства альдегидов
Химические свойства альдегидов Роль металлов в истории человеческой цивилизации
Роль металлов в истории человеческой цивилизации Современные химические технологии
Современные химические технологии Металлы. Общая характеристика
Металлы. Общая характеристика Алотропні модифікації карбону
Алотропні модифікації карбону  Продукт полимеризации стирола - полистирол
Продукт полимеризации стирола - полистирол Арены. Бензол
Арены. Бензол Оксиды. Классификация. Получение. Свойства
Оксиды. Классификация. Получение. Свойства Основы составления балансов
Основы составления балансов Фотосинтез. Суммарное уравнение, общий вид
Фотосинтез. Суммарное уравнение, общий вид Алкины. Характеристика тройной связи
Алкины. Характеристика тройной связи Классификация органических веществ. Автор: Русакова А.В. учитель химии МОУ «Гимназия № 19» г. Омска.
Классификация органических веществ. Автор: Русакова А.В. учитель химии МОУ «Гимназия № 19» г. Омска.  Биологически значимые элементы
Биологически значимые элементы Галогены
Галогены Олиго- и гомополисахариды
Олиго- и гомополисахариды Альдегиды. (10 класс)
Альдегиды. (10 класс) Геохимические методы исследований
Геохимические методы исследований Презентация по Химии "Углекислый газ" - скачать смотреть
Презентация по Химии "Углекислый газ" - скачать смотреть  Биологические мембраны. Структурная организация
Биологические мембраны. Структурная организация Почвенный раствор. Химический состав почвенных растворов. Водный режим почв. Кислотность и щелочность почвенных растворов
Почвенный раствор. Химический состав почвенных растворов. Водный режим почв. Кислотность и щелочность почвенных растворов Топлива, применяемые на воздушных судах и наземной технике. Эксплуатационные свойства топлив. (Тема 2.1)
Топлива, применяемые на воздушных судах и наземной технике. Эксплуатационные свойства топлив. (Тема 2.1) Витамин В2 (рибофлавин)
Витамин В2 (рибофлавин)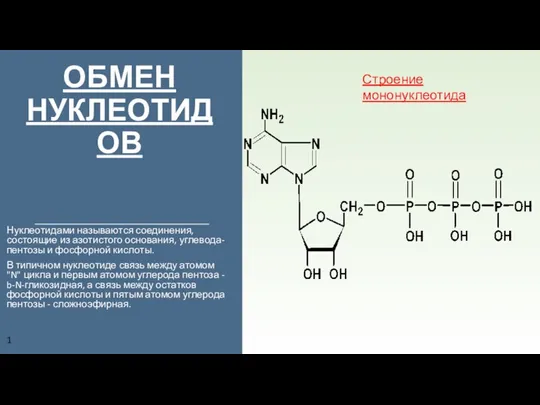 Обмен нуклеотидов. Строение мононуклеотида
Обмен нуклеотидов. Строение мононуклеотида Аминокислоты. Белки. Пептиды
Аминокислоты. Белки. Пептиды Сопротивление материалов. Металлический тип химической связи и основные свойства металлов
Сопротивление материалов. Металлический тип химической связи и основные свойства металлов