Содержание
- 2. Plan: 1. Definition about mercury; 2. Etymology of mercury; 3. Properties; 4. Occurrence; 5. Applications; 6.
- 4. Mercury is a chemical element with symbol Hg and atomic number 80. It is commonly known
- 5. A heavy, silvery d-block element, mercury is the only metallic element that is liquid at standard
- 6. Mercury occurs in deposits throughout the world mostly as cinnabar (mercuric sulfide). The red pigment vermilion
- 8. Etymology Hg is the modern chemical symbol for mercury. It comes from hydrargyrum, a Latinized form
- 9. Properties Physical properties Mercury is a heavy, silvery-white liquid metal. Compared to other metals, it is
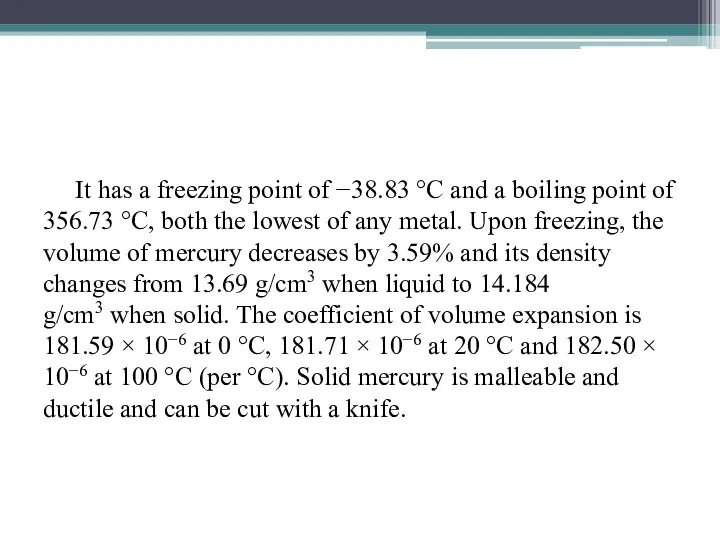
- 10. It has a freezing point of −38.83 °C and a boiling point of 356.73 °C, both
- 12. Chemical properties Mercury does not react with most acids, such as dilute sulfuric acid, although oxidizing
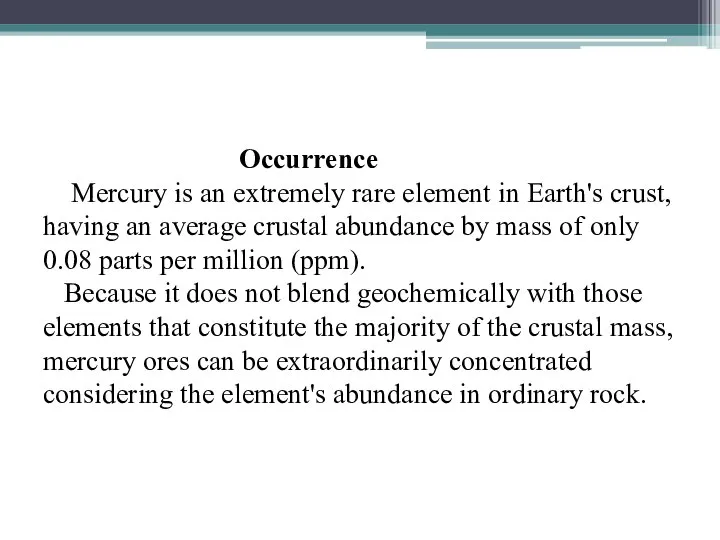
- 13. Occurrence Mercury is an extremely rare element in Earth's crust, having an average crustal abundance by
- 14. Applications Mercury is used primarily for the manufacture of industrial chemicals or for electrical and electronic
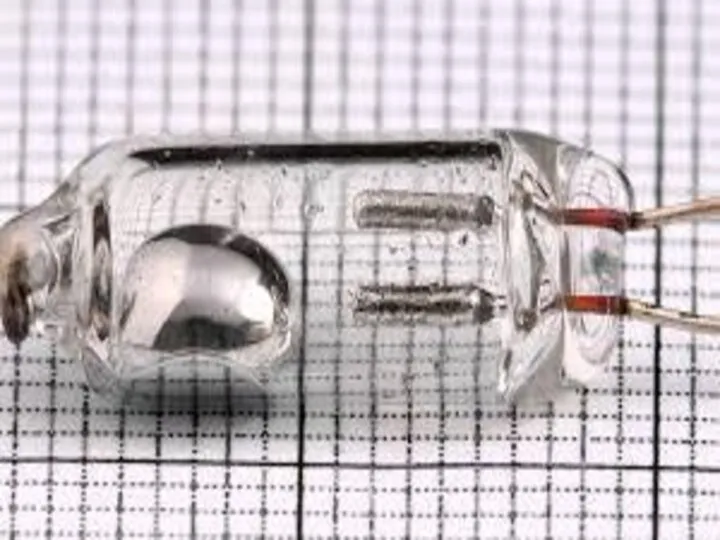
- 15. The bulb of a mercury-in-glass thermometer
- 16. Medicine Mercury and its compounds have been used in medicine, although they are much less common
- 17. Amalgam filling
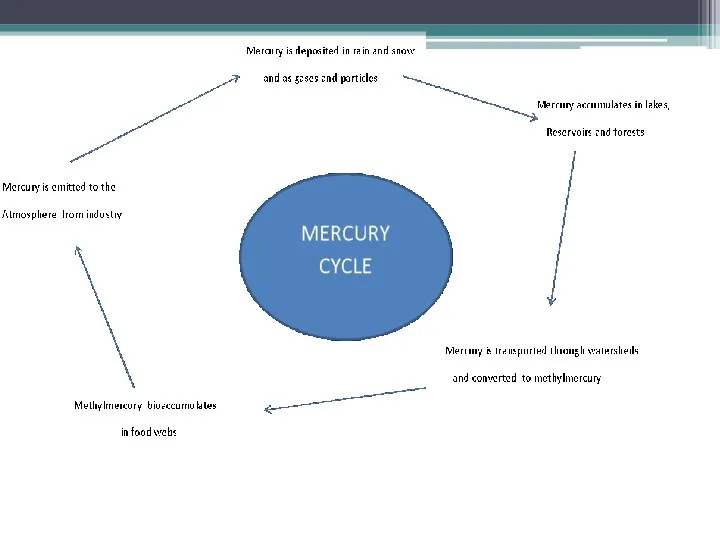
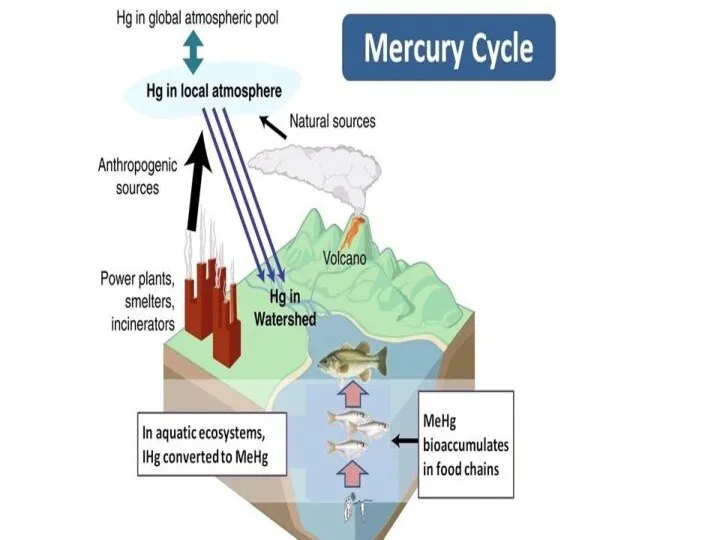
- 18. Mercury cycle The mercury cycle is a biogeochemical cycle involving mercury. Mercury is notable for being
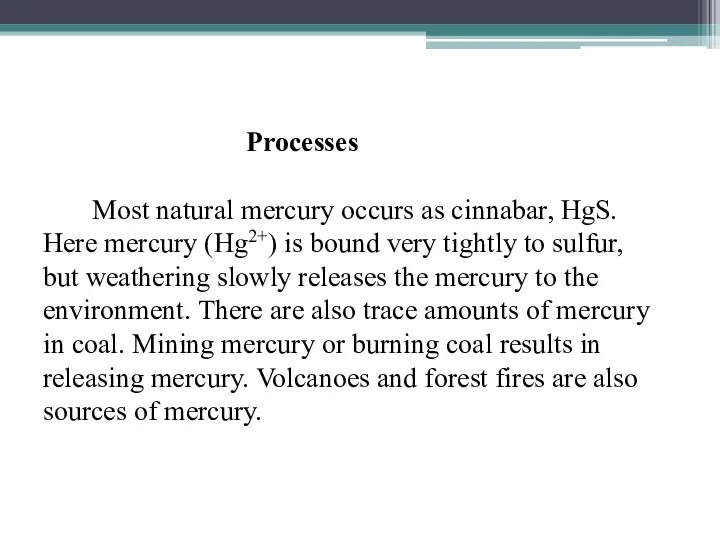
- 21. Processes Most natural mercury occurs as cinnabar, HgS. Here mercury (Hg2+) is bound very tightly to
- 22. Chlorine factories, among other sources, release mercury into the atmosphere. This mercury is deposited back onto
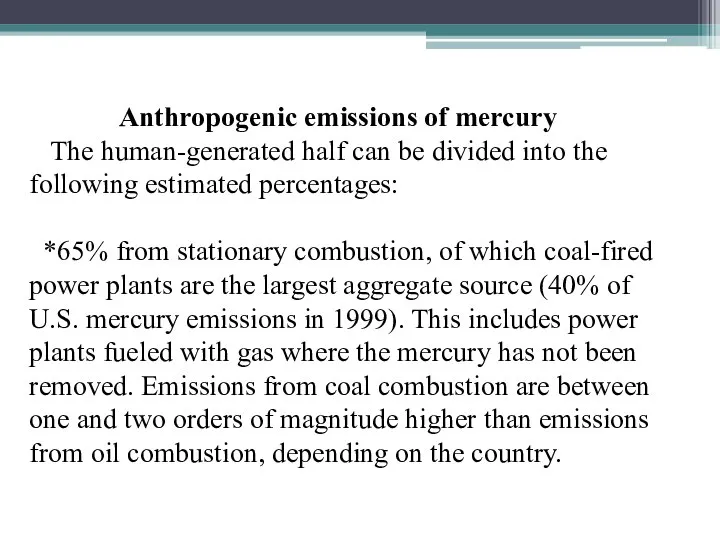
- 23. Anthropogenic emissions of mercury The human-generated half can be divided into the following estimated percentages: *65%
- 24. *11% from gold production. The three largest point sources for mercury emissions in the U.S. are
- 26. Скачать презентацию
















 Органическая химия
Органическая химия Кислоты. Классификация кислот
Кислоты. Классификация кислот Трифенилметановые красители
Трифенилметановые красители Введение. Виды стекол. Применение конструкций из стекла
Введение. Виды стекол. Применение конструкций из стекла Atomic structure
Atomic structure Основные понятия и законы химии
Основные понятия и законы химии Презентация по Химии "Синтетические лекарственные средства" - скачать смотреть бесплатно
Презентация по Химии "Синтетические лекарственные средства" - скачать смотреть бесплатно Химические свойства
Химические свойства Общие свойства металлов. Сплавы
Общие свойства металлов. Сплавы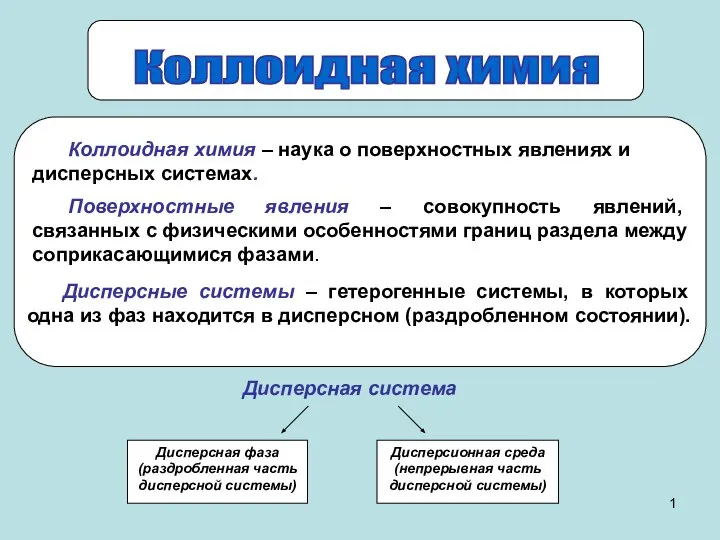 Презентация на тему "Коллоидная химия"
Презентация на тему "Коллоидная химия" Прикладная химия. Теплоперенос в химических реакторах и теплообменники
Прикладная химия. Теплоперенос в химических реакторах и теплообменники Химия в криминалистике
Химия в криминалистике Պոլիմերներ
Պոլիմերներ Феноло-альдегидные олигомеры и пластмассы на их основе
Феноло-альдегидные олигомеры и пластмассы на их основе Минералы и их строение
Минералы и их строение Строение вещества. Химическая связь
Строение вещества. Химическая связь Качественные реакции. Катионы
Качественные реакции. Катионы ГИА. А1: Строение атомов первых 20 химических элементов ПСХЭ
ГИА. А1: Строение атомов первых 20 химических элементов ПСХЭ Группа щелочных габброидов
Группа щелочных габброидов Хімія як наука
Хімія як наука Презентация по Химии "Хлор" - скачать смотреть
Презентация по Химии "Хлор" - скачать смотреть  Натуральні і синтетичні каучуки Виконала: Учениця 11 класу Пристинська Ірина .
Натуральні і синтетичні каучуки Виконала: Учениця 11 класу Пристинська Ірина .  Презентация Окислительно-восстановительные реакции
Презентация Окислительно-восстановительные реакции Правила ДСС
Правила ДСС Явище ізомерії. Структурна ізомерія. Близнюки органічного світу
Явище ізомерії. Структурна ізомерія. Близнюки органічного світу Ванадий
Ванадий Термический анализ
Термический анализ Химический состав костей
Химический состав костей