Содержание
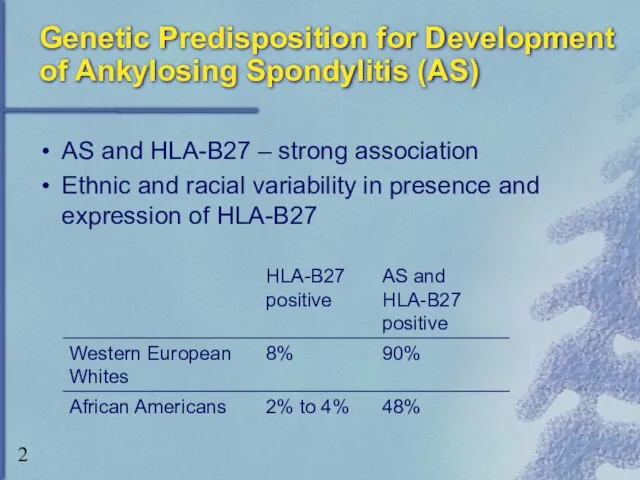
- 2. Genetic Predisposition for Development of Ankylosing Spondylitis (AS) AS and HLA-B27 – strong association Ethnic and
- 3. Natural History of AS Highly variable Early stages: spontaneous remissions and exacerbations Spectrum of severity Mild
- 4. Burden of Illness Functional disability Potential complications Quality-of-life issues Pain, stiffness, fatigue, sleep problems Healthcare costs
- 5. Obstacles to Desirable Outcomes in AS Until Recently Diagnostic and classification limitations Lack of universally accepted
- 6. Advances in Medicine: Hope for Patients With AS Increased understanding of pathophysiologic processes Advent of Anti-TNF
- 7. Pathogenesis of AS Incompletely understood, but knowledge increasing Interaction between HLA-B27 and T-cell response Increased concentration
- 8. Clinical Features of AS
- 9. Modified New York Criteria for the Diagnosis of AS Clinical Criteria Low back pain, > 3
- 10. Disease Activity Assessment BASFI = Bath Ankylosing Spondylitis Functional Index BASDAI = Bath Ankylosing Spondylitis Disease
- 11. Bath Ankylosing Spondylitis Functional Index (BASFI) Visual analog scale (VAS) – 10 cm Mean score of
- 12. Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) A self-administered instrument (using 10-cm horizontal visual analog scales)
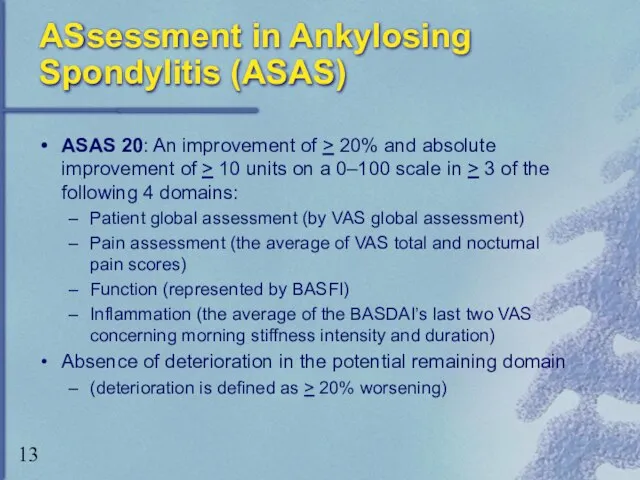
- 13. ASsessment in Ankylosing Spondylitis (ASAS) ASAS 20: An improvement of > 20% and absolute improvement of
- 14. Introduction of Anti-TNF Agents for the Treatment of Ankylosing Spondylitis US Modifications of the ASAS International
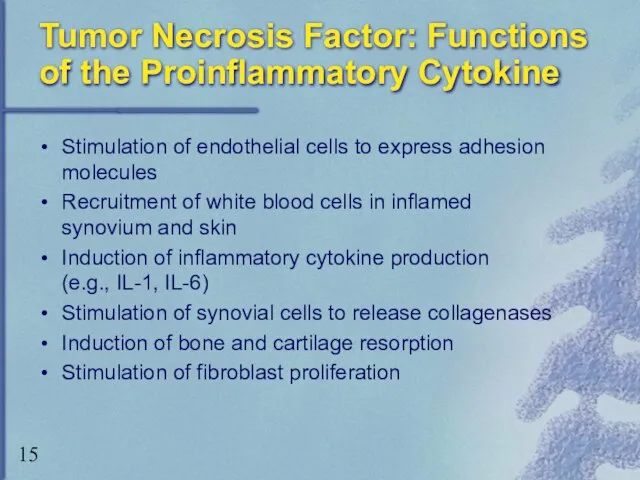
- 15. Tumor Necrosis Factor: Functions of the Proinflammatory Cytokine Stimulation of endothelial cells to express adhesion molecules
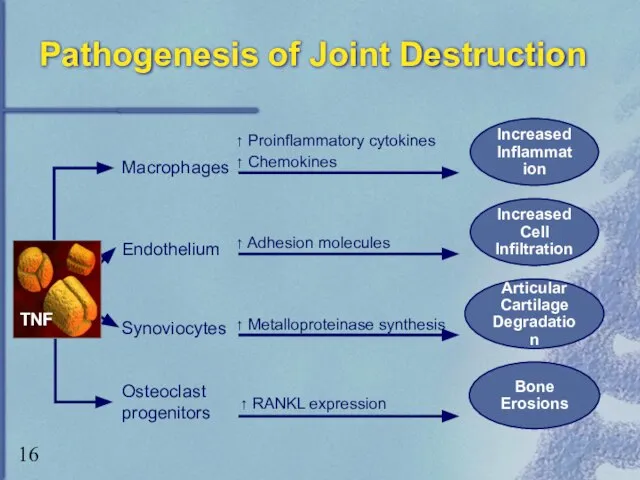
- 16. Pathogenesis of Joint Destruction Bone Erosions Macrophages Endothelium Synoviocytes ↑ Proinflammatory cytokines ↑ Chemokines ↑ Adhesion
- 17. US Modifications of the ASAS International Guidelines: Appropriate Patients for Anti-TNF Therapy Definitive AS according to
- 18. Contraindications for Anti-TNF Therapy Current or recurrent infections Tuberculosis Multiple sclerosis Lupus Malignancy Pregnant or lactating
- 19. Monitoring and Discontinuing Treatment With Anti-TNF Agents ASAS core set of outcome parameters to monitor patients
- 20. Anti-TNF Agents Etanercept Approved in the United States and Europe for treatment of AS Dose: 50
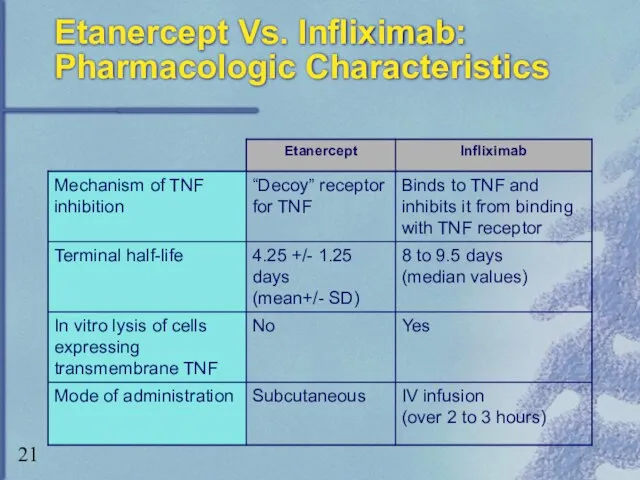
- 21. Etanercept Vs. Infliximab: Pharmacologic Characteristics
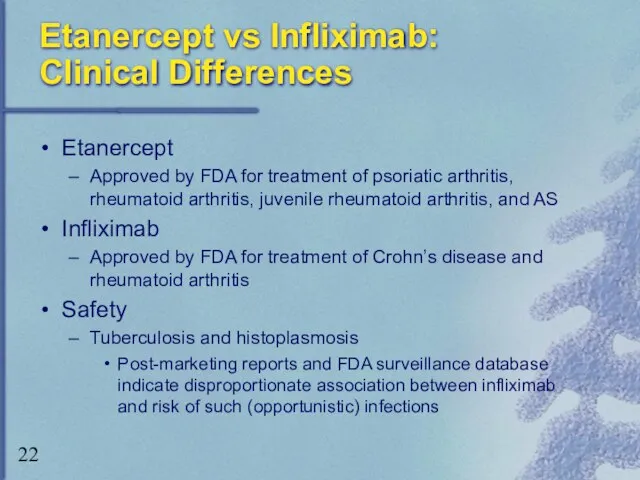
- 22. Etanercept vs Infliximab: Clinical Differences Etanercept Approved by FDA for treatment of psoriatic arthritis, rheumatoid arthritis,
- 23. Etanercept for the Treatment of AS: Clinical Trials Marzo-Ortega, et al. Significant improvement in all clinical
- 24. Etanercept for the Treatment of AS: Clinical Trials (cont) Brandt, et al. 57% etanercept-treated patients and
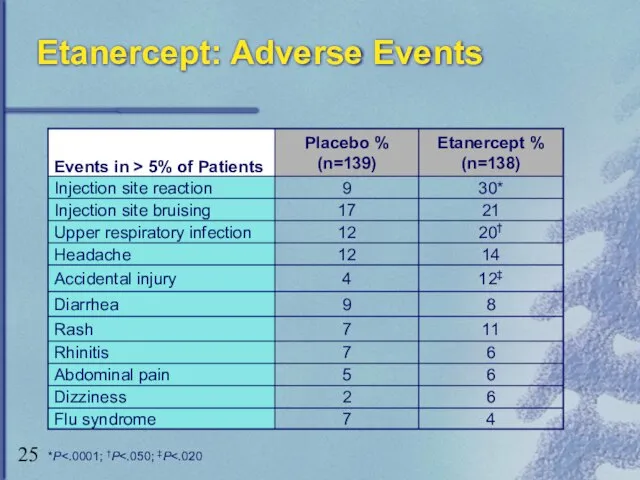
- 25. Etanercept: Adverse Events *P
- 26. Etanercept: Adverse Events (cont) Serious infections and sepsis Mainly in patients with underlying illness or receiving
- 27. Infliximab for the Treatment of AS: Clinical Trials Brandt, et al. ≥ 50% improvement on outcome
- 28. Infliximab for the Treatment of AS: Clinical Trials (cont) Stone, et al. Improvement of > 60%
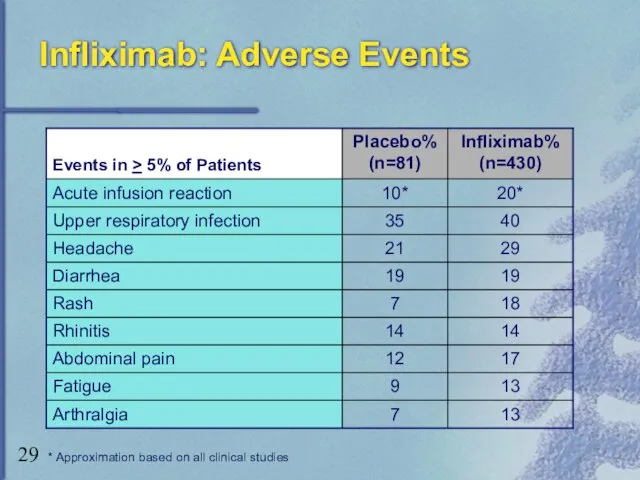
- 29. Infliximab: Adverse Events * Approximation based on all clinical studies
- 30. Infliximab: Adverse Events (cont) Serious infections and sepsis Cases in patients on concomitant immunosuppressive therapy Neurologic
- 31. Anti-TNF Agents: Summary Anti-TNF agents target underlying inflammatory process Alter disease progression Provide symptomatic relief Recommended
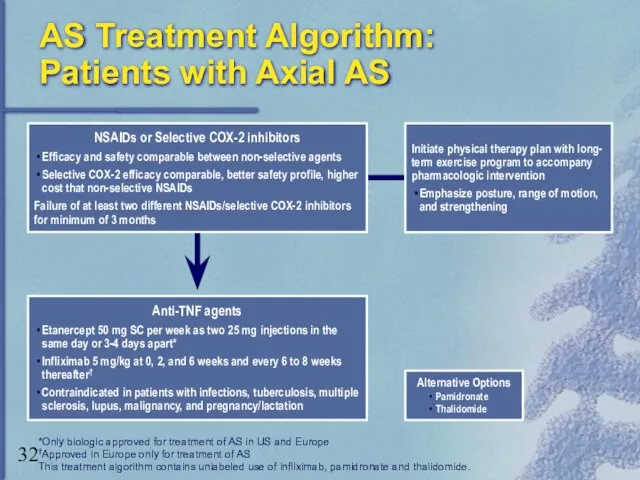
- 32. AS Treatment Algorithm: Patients with Axial AS Alternative Options Pamidronate Thalidomide *Only biologic approved for treatment
- 34. Скачать презентацию






















 Анатомия, физиология и патология органов речи
Анатомия, физиология и патология органов речи Нарушения обмена углеводов
Нарушения обмена углеводов Жедел панкреатит емдеу тәсілдері
Жедел панкреатит емдеу тәсілдері Менің жан дұнием.Таным
Менің жан дұнием.Таным Геморрагический шок в гинекологии
Геморрагический шок в гинекологии Як можна зберегти здоров’я в умовах сучасної екології. Франція
Як можна зберегти здоров’я в умовах сучасної екології. Франція Понятие о клинической смерти. Правила обращения с трупом
Понятие о клинической смерти. Правила обращения с трупом Теория поглаживаний
Теория поглаживаний Белково-калорийная недостаточность и её последствия. Пищевые аллергии. Аллергия на глютен
Белково-калорийная недостаточность и её последствия. Пищевые аллергии. Аллергия на глютен Школа сахарного диабета
Школа сахарного диабета Новые возможности органосохраняющего лечения миомы матки
Новые возможности органосохраняющего лечения миомы матки Бактериофаги - враги наших врагов
Бактериофаги - враги наших врагов Пороки развития позвонков и врожденные деформации позвоночника
Пороки развития позвонков и врожденные деформации позвоночника Хирургиялық стоматологиядағы дәлелді медецина
Хирургиялық стоматологиядағы дәлелді медецина Нормальная артикуляция. Согласные звуки
Нормальная артикуляция. Согласные звуки Аудиометрия. Звуковые методы исследования в медицине: перкуссия, аускультация. Фонокардиография
Аудиометрия. Звуковые методы исследования в медицине: перкуссия, аускультация. Фонокардиография Учение об эпидемическом процессе. Организация профилактических и противоэпидемических мероприятий
Учение об эпидемическом процессе. Организация профилактических и противоэпидемических мероприятий Первая помощь при инфаркте и инсульте
Первая помощь при инфаркте и инсульте Психологические особенности детей с нарушениями зрения
Психологические особенности детей с нарушениями зрения Масштаб проблемы ВБИ. Структура ВБИ
Масштаб проблемы ВБИ. Структура ВБИ Аса қауіпті инфекциялардағы профилактикалық шаралар мен алгоритмдер
Аса қауіпті инфекциялардағы профилактикалық шаралар мен алгоритмдер Анатомическое строение треугольника Пти
Анатомическое строение треугольника Пти Су-электролиттік бұзылыстар және инфузиялық терапияның принциптері
Су-электролиттік бұзылыстар және инфузиялық терапияның принциптері Возбудитель скарлатины
Возбудитель скарлатины Как эффективно начать и убедительно закончить выступление
Как эффективно начать и убедительно закончить выступление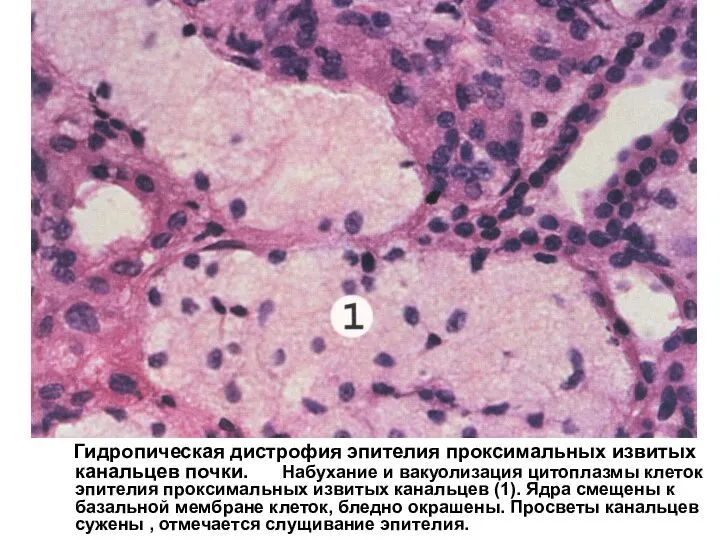 Гидропическая дистрофия эпителия проксимальных извитых канальцев почки
Гидропическая дистрофия эпителия проксимальных извитых канальцев почки Құрсақ ішілік инфекциялар. Қызамық
Құрсақ ішілік инфекциялар. Қызамық Личность в теории Эрика Берна
Личность в теории Эрика Берна