Good Pharmacovigilance Practice (GVP) of the Eurasian Economic Union, approved by
the Decision of the Council of the Eurasian Economic Commission No. 87 of 03/11/2016 (entered into force on 06.05.2017)
Federal Law No. 61-FZ of April 12, 2010, About the Circulation of Medicinal Products (Chapter 13)
Law of the Ministry of Health of Russia No. 682n of 09/07/2016 “On approval of the form of the document containing the results of monitoring the effectiveness and safety of medicinal products for medical use, carried out by the holder or owner of the registration certificate of the medicinal products or an authorized legal entity”
Order of Roszdravnadzor No. 1071 of February 15, 2017 “On Approval of the Procedure for the Implementation of Pharmacovigilance”
Order of the Ministry of Health of Russia dated 07.09.2015 No. 5539 “On approval of the procedure for the implementation of selective quality control of drugs for medical use”
The Main Legal Acts in PV






 Православное христианство в истории России. Часть 2
Православное христианство в истории России. Часть 2 Основы программирования. Указатели и динамические массивы
Основы программирования. Указатели и динамические массивы Метод интервалов Подготовила: учитель математики МОУ сош №30 имени А.И.Колдунова Кутоманова Е.М. 2010-2011 учебный год
Метод интервалов Подготовила: учитель математики МОУ сош №30 имени А.И.Колдунова Кутоманова Е.М. 2010-2011 учебный год ГОУ ВПО «Красноярский Государственный Медицинский Университет имени профессора В. Ф. Войно-Ясенецкого Министерства здравоохр
ГОУ ВПО «Красноярский Государственный Медицинский Университет имени профессора В. Ф. Войно-Ясенецкого Министерства здравоохр Обряд освящения - оваа
Обряд освящения - оваа Презентация Предпосылки образования Древнерусского государства
Презентация Предпосылки образования Древнерусского государства  Структура и состав культурологического знания
Структура и состав культурологического знания Виды принтеров
Виды принтеров Цель и содержание обучения ИЯ
Цель и содержание обучения ИЯ Презентация ВАЖНЕЙШИЕ ФАКТОРЫ РАЗВИТИЯ УПРАВЛЕНЧЕСКОЙ МЫСЛИ В РОССИИ XVII в
Презентация ВАЖНЕЙШИЕ ФАКТОРЫ РАЗВИТИЯ УПРАВЛЕНЧЕСКОЙ МЫСЛИ В РОССИИ XVII в Финансы и кредит Денежная система
Финансы и кредит Денежная система РЕТТЕУШІ ОЛИГОПЕПТИДТЕР ҚЫЗМЕТІ ОРЫНДАҒАН:РАХАТ ГҮЛНАЗ ФАКУЛЬТЕТ:ЖМ ТОБЫ:16/1Қ
РЕТТЕУШІ ОЛИГОПЕПТИДТЕР ҚЫЗМЕТІ ОРЫНДАҒАН:РАХАТ ГҮЛНАЗ ФАКУЛЬТЕТ:ЖМ ТОБЫ:16/1Қ Стиль Рококо – возникновение и особенности
Стиль Рококо – возникновение и особенности Порядок подготовки проекта правил землепользования застройки. Лекция 4
Порядок подготовки проекта правил землепользования застройки. Лекция 4 Электрические плиты
Электрические плиты 33 богатыря - презентация для начальной школы
33 богатыря - презентация для начальной школы Особенности развития современной политической системы
Особенности развития современной политической системы Стресс, фрустрация
Стресс, фрустрация Характеристика объектов автоматизации сельскохозяйственного производства
Характеристика объектов автоматизации сельскохозяйственного производства Презентация на тему "Требования к домашнему заданию" - скачать презентации по Педагогике
Презентация на тему "Требования к домашнему заданию" - скачать презентации по Педагогике Язык программирования Паскаль
Язык программирования Паскаль ИСТОРИЯ ГОСУДАРСТВА И ПРАВА ЗАРУБЕЖНЫХ СТРАН
ИСТОРИЯ ГОСУДАРСТВА И ПРАВА ЗАРУБЕЖНЫХ СТРАН 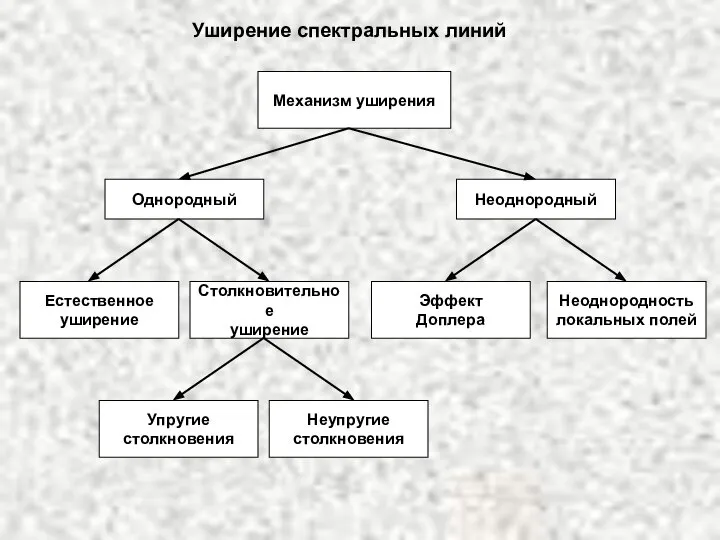 Уширение спектральных линий
Уширение спектральных линий  Дымковская игрушка
Дымковская игрушка Инфекция кожи и подкожной клетчатки
Инфекция кожи и подкожной клетчатки Типы двигателей
Типы двигателей Игристое вино
Игристое вино Цикл внутреннего аудита
Цикл внутреннего аудита