Содержание
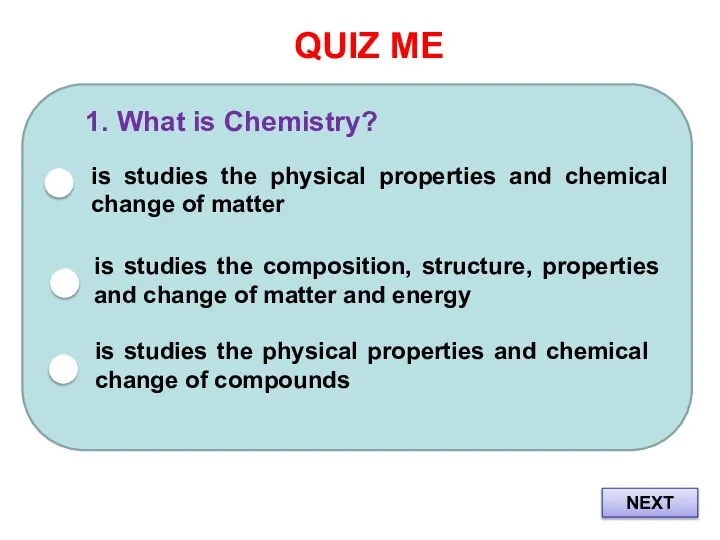
- 2. QUIZ ME 1. What is Chemistry? is studies the physical properties and chemical change of matter
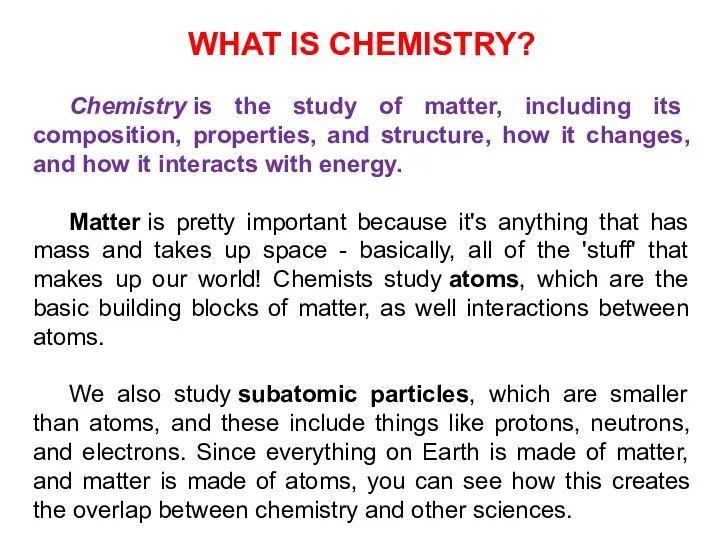
- 3. WHAT IS CHEMISTRY? Chemistry is the study of matter, including its composition, properties, and structure, how
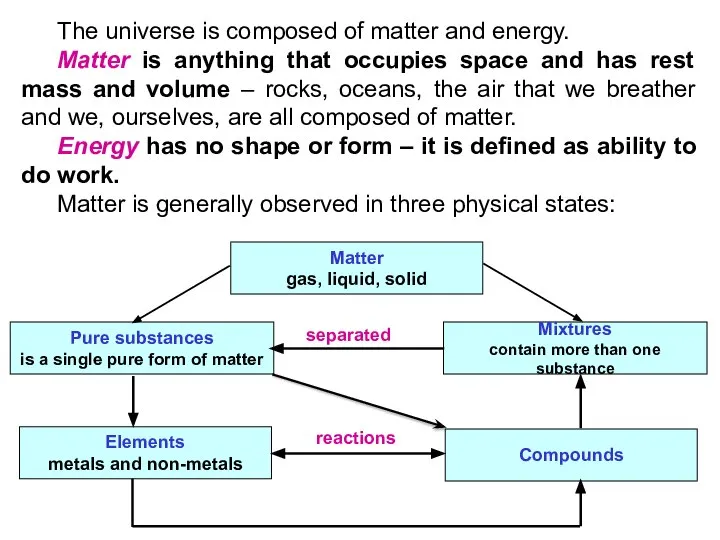
- 4. The universe is composed of matter and energy. Matter is anything that occupies space and has
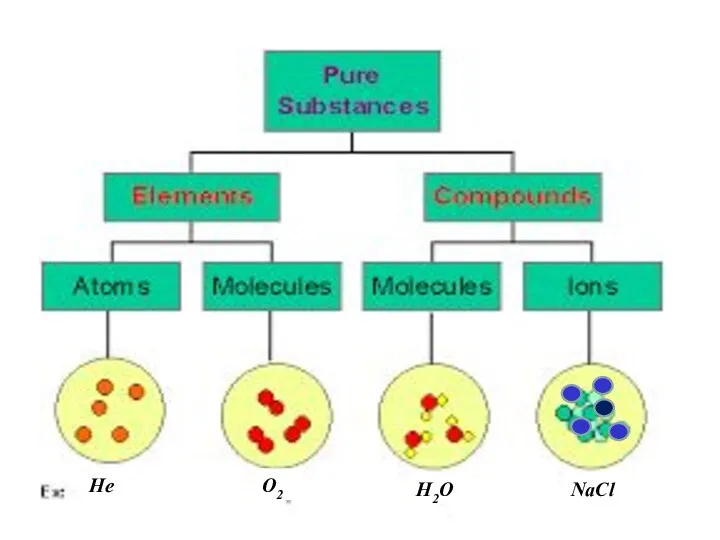
- 5. He O2 H2O NaCl
- 6. QUIZ ME 2. A pure substance can only be: a heterogeneous mixture an element or a
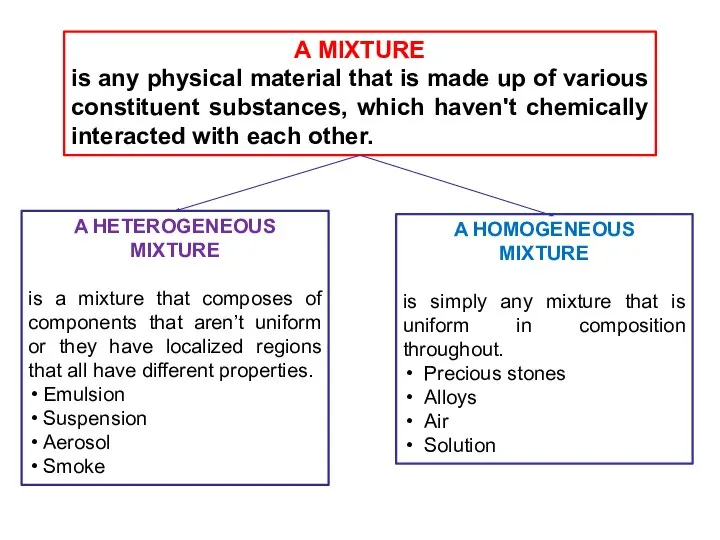
- 7. A MIXTURE is any physical material that is made up of various constituent substances, which haven't
- 8. QUIZ ME 3. Which one of the following mixture is homogeneous? starch and sugar ethanol and
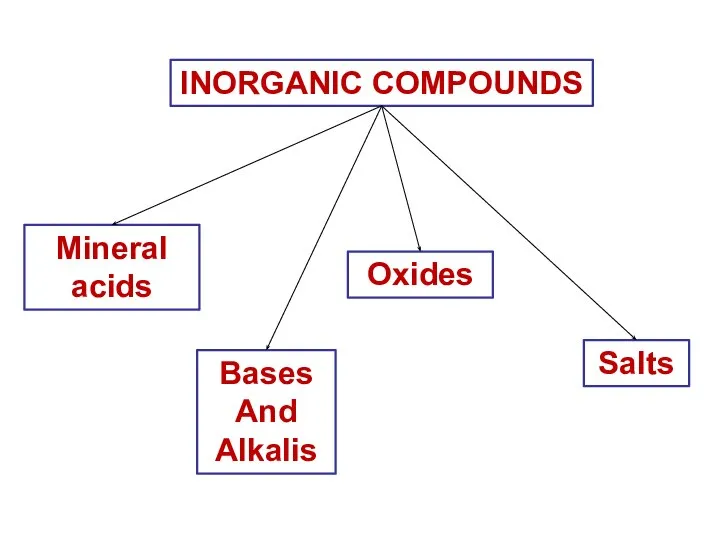
- 9. INORGANIC COMPOUNDS Mineral acids Bases And Alkalis Oxides Salts
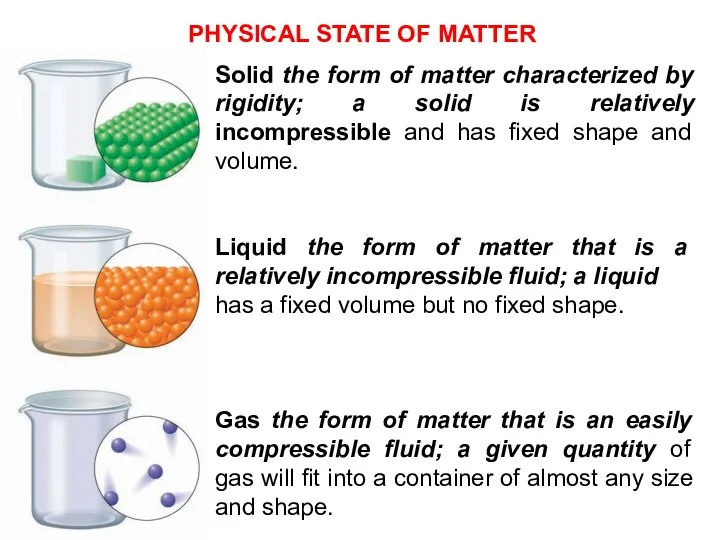
- 10. Solid the form of matter characterized by rigidity; a solid is relatively incompressible and has fixed
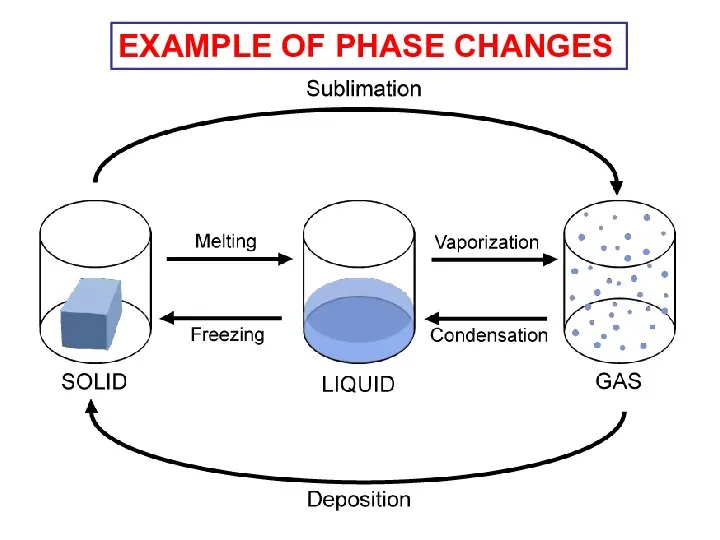
- 11. EXAMPLE OF PHASE CHANGES
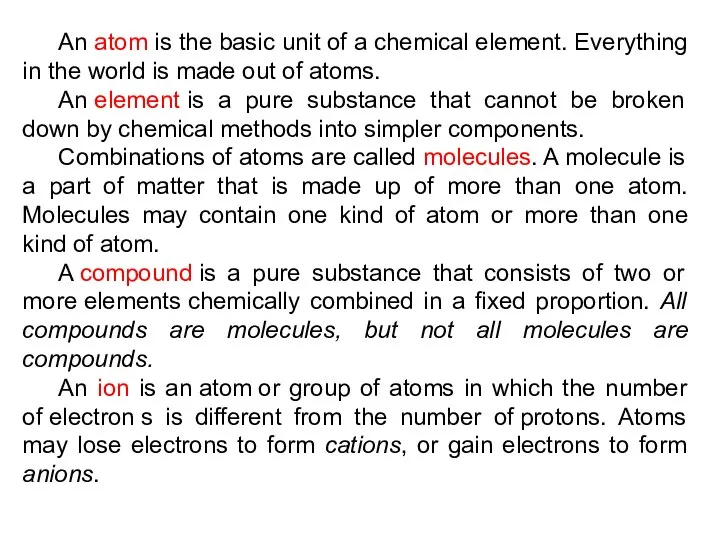
- 13. An atom is the basic unit of a chemical element. Everything in the world is made
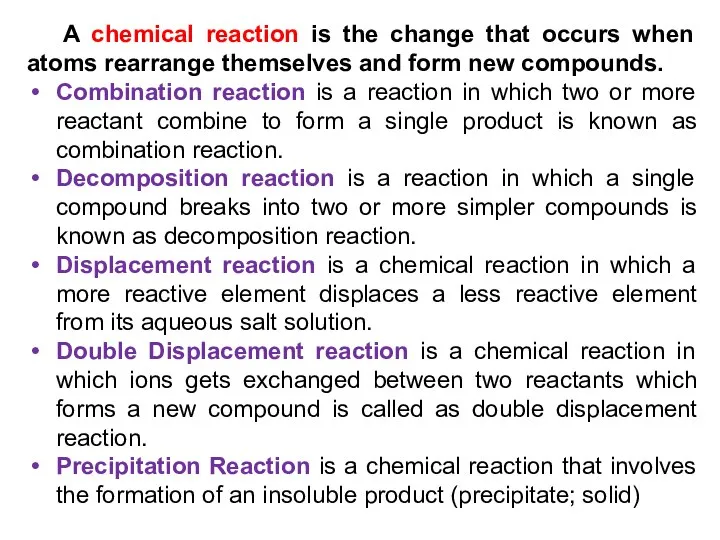
- 14. A chemical reaction is the change that occurs when atoms rearrange themselves and form new compounds.
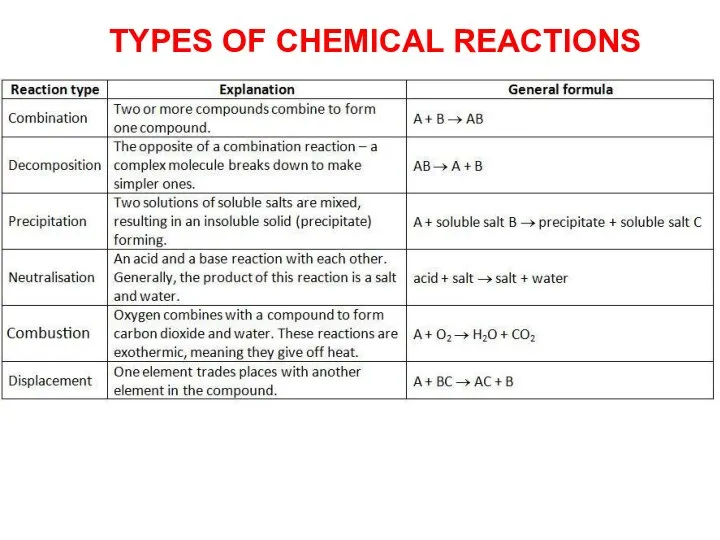
- 15. TYPES OF CHEMICAL REACTIONS
- 17. QUIZ ME 4. The father of modern chemistry is: Dalton Lavoisier Mendeleeff NEXT Proust
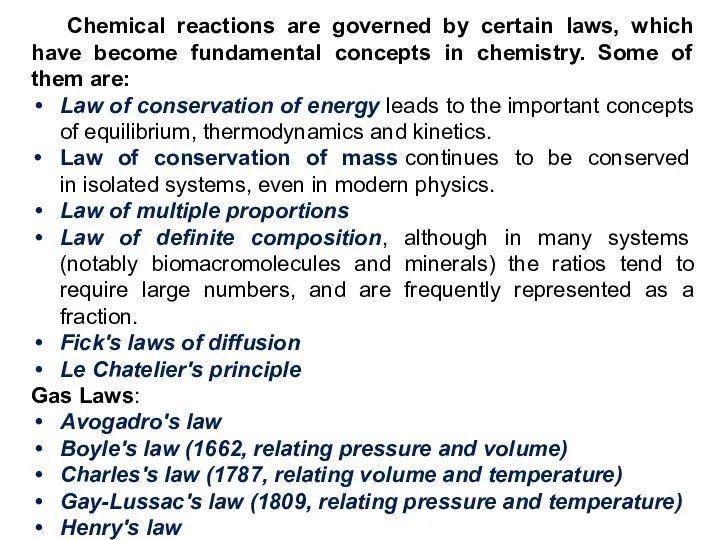
- 18. Chemical reactions are governed by certain laws, which have become fundamental concepts in chemistry. Some of
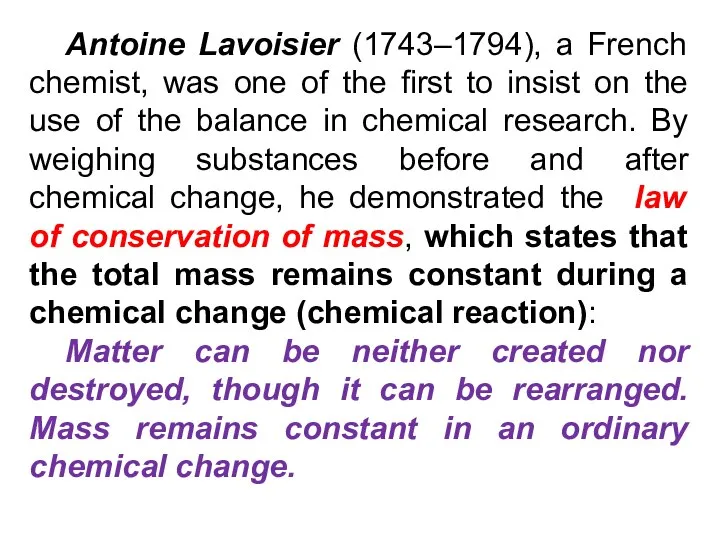
- 19. Antoine Lavoisier (1743–1794), a French chemist, was one of the first to insist on the use
- 20. 2*200 + 32 = 2*(200+16) 432 = 432
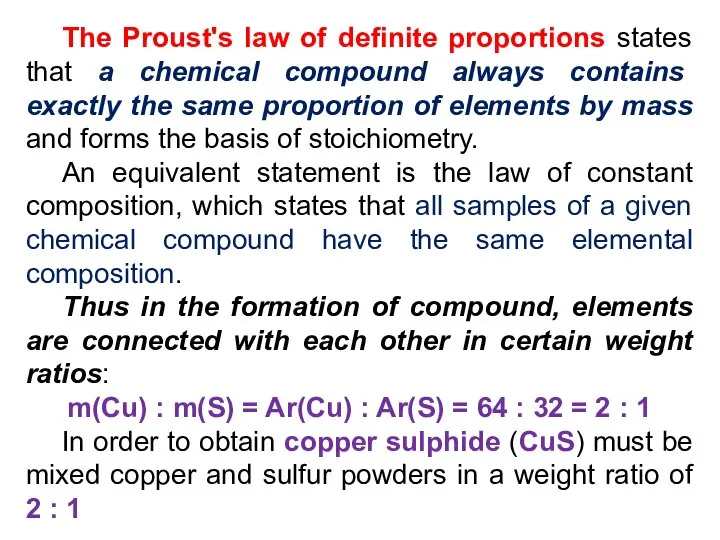
- 21. The Proust's law of definite proportions states that a chemical compound always contains exactly the same
- 22. DALTON'S ATOMIC THEORY John Dalton (1808) used the Greek concept of an atom and the laws
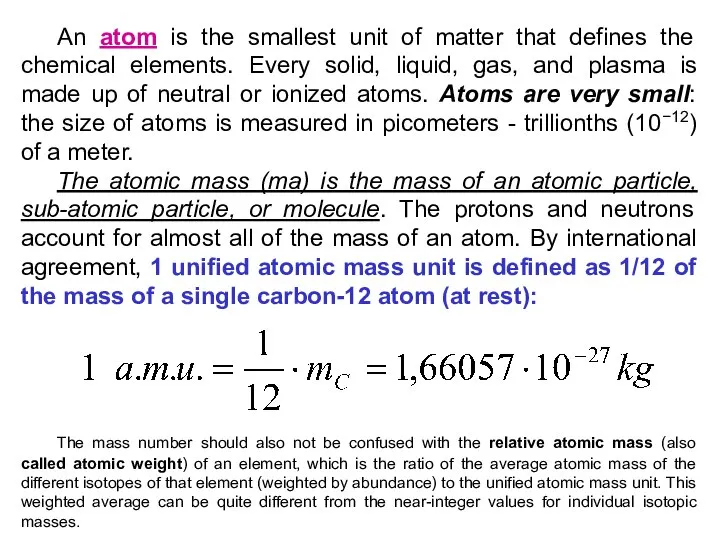
- 23. An atom is the smallest unit of matter that defines the chemical elements. Every solid, liquid,
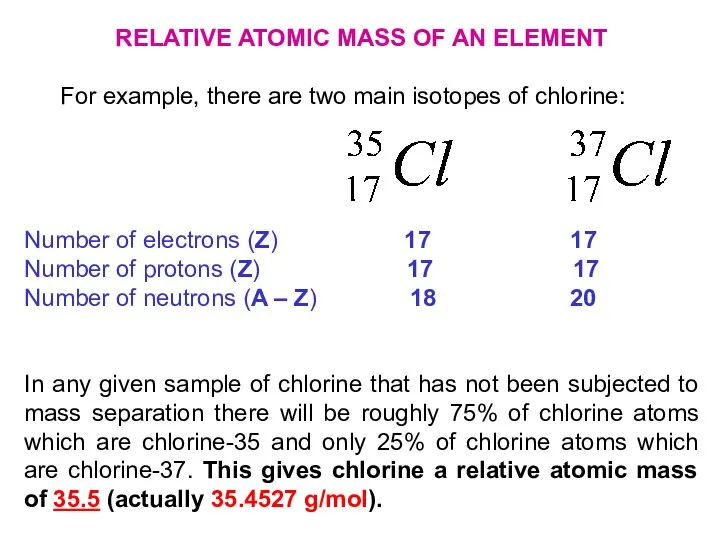
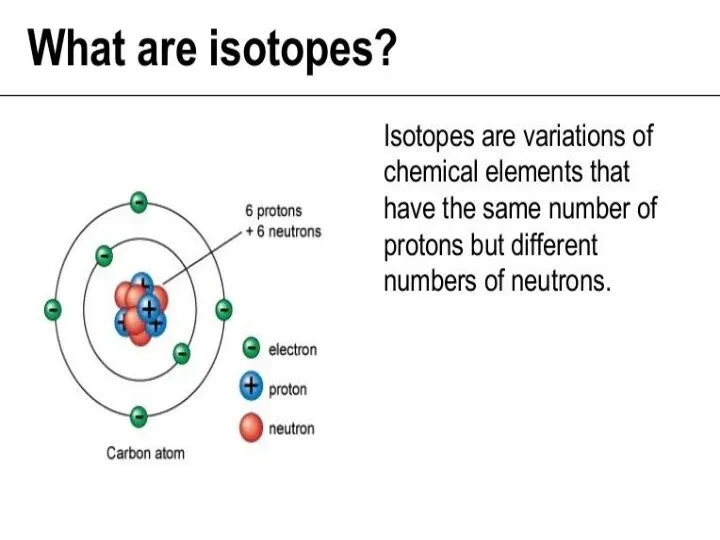
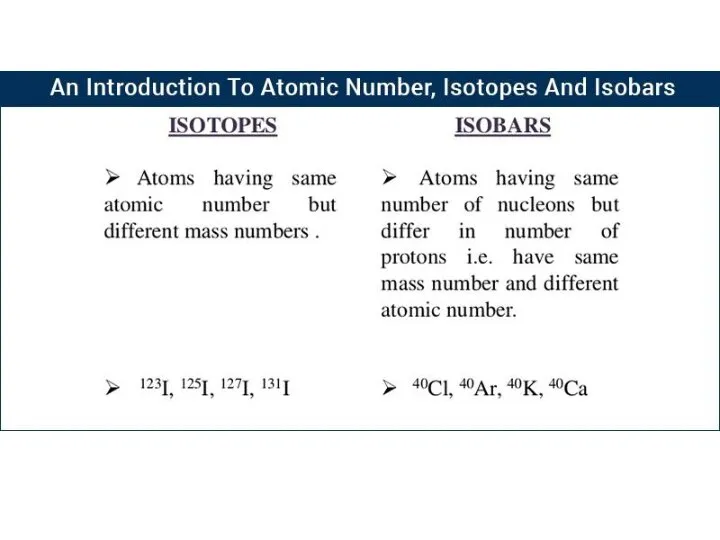
- 24. RELATIVE ATOMIC MASS OF AN ELEMENT For example, there are two main isotopes of chlorine: Number
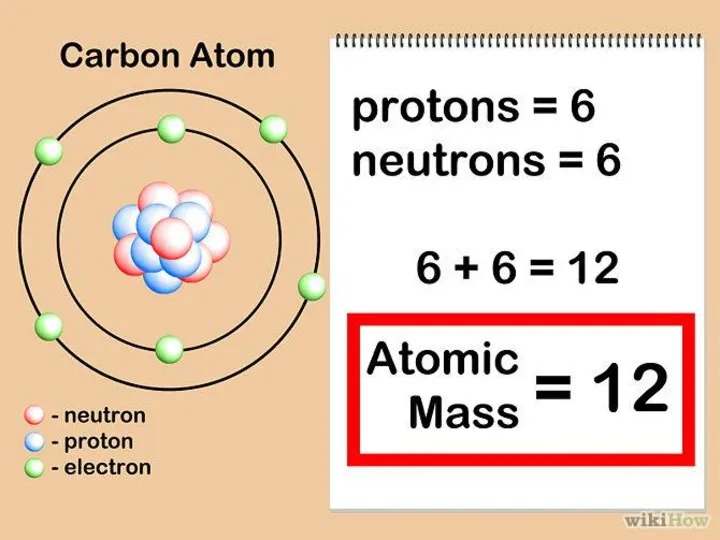
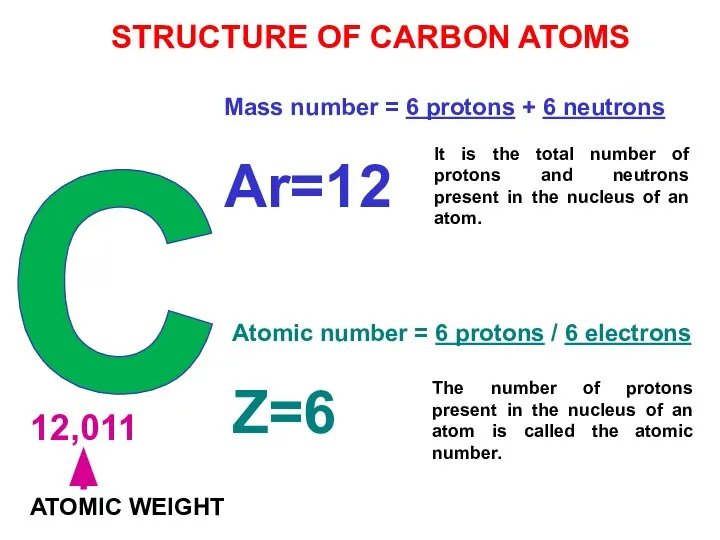
- 26. C 12,011 ATOMIC WEIGHT Mass number = 6 protons + 6 neutrons Ar=12 Atomic number =
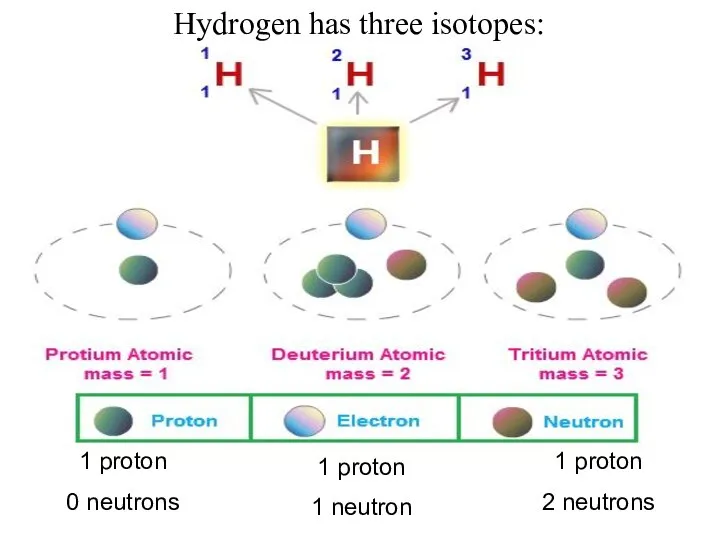
- 28. Hydrogen has three isotopes: 1 proton 0 neutrons 1 proton 1 neutron 1 proton 2 neutrons
- 30. CHEMICAL BOND The sharing or transfer of electrons creates some attraction force between elements that is
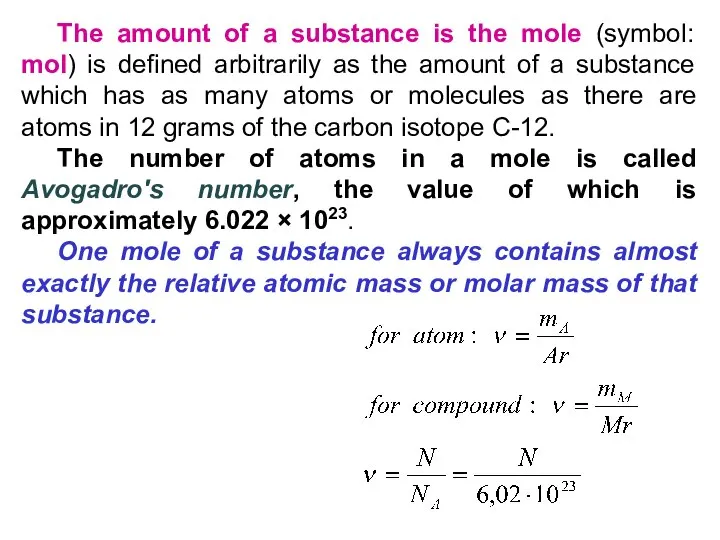
- 31. The amount of a substance is the mole (symbol: mol) is defined arbitrarily as the amount
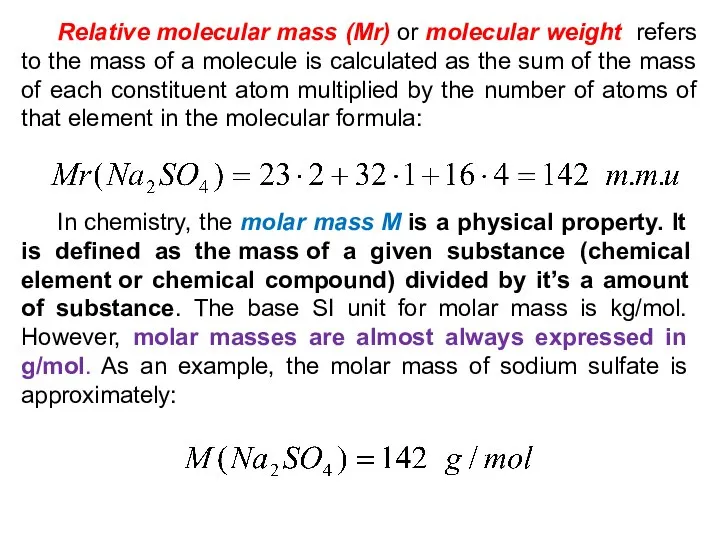
- 32. Relative molecular mass (Mr) or molecular weight refers to the mass of a molecule is calculated
- 33. Even before the creation of the doctrine of atom and molecula it was found that simple
- 34. The equivalent (symbol: Eq), sometimes termed the molar equivalent, is a unit of electrical charge used
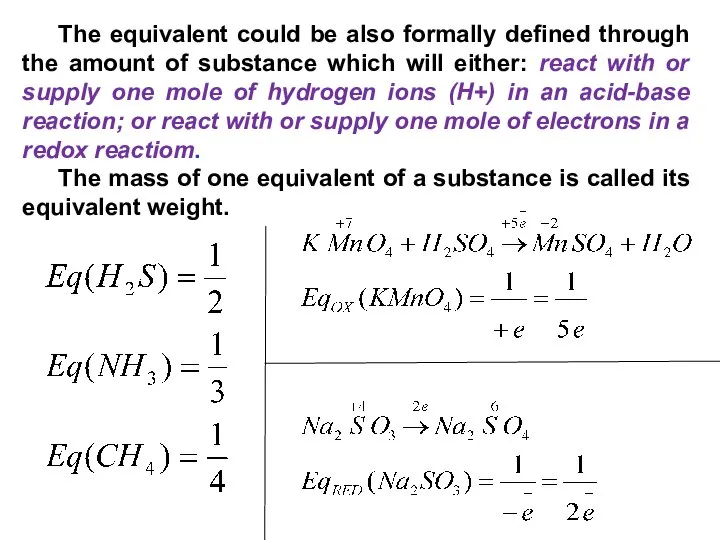
- 35. The equivalent could be also formally defined through the amount of substance which will either: react
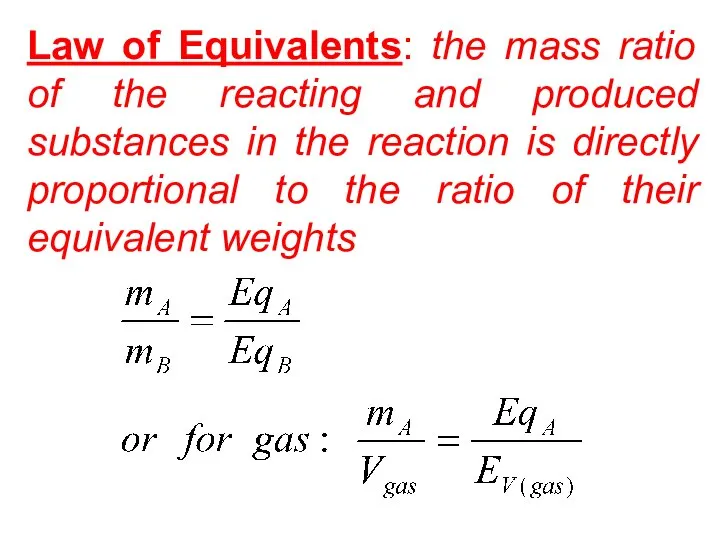
- 37. Law of Equivalents: the mass ratio of the reacting and produced substances in the reaction is
- 38. Avogadro's Law Amedeo Avogadro introduced the term "molecule" and distinguished it from 'atom'. According to Avogadro,
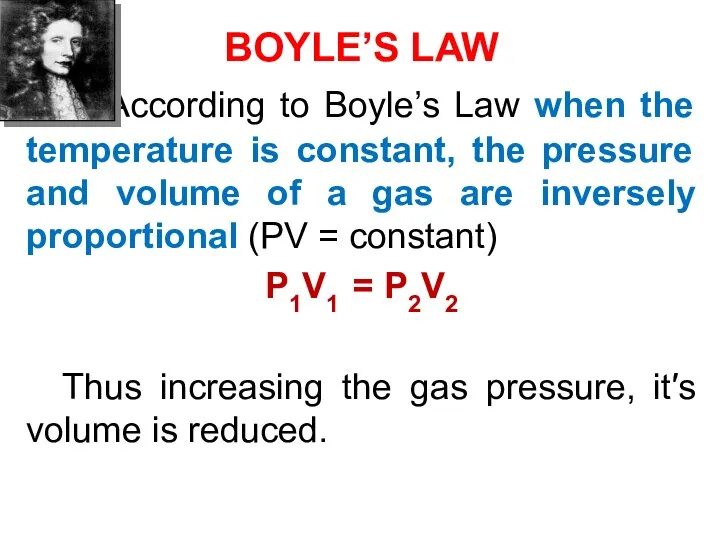
- 39. BOYLE’S LAW According to Boyle’s Law when the temperature is constant, the pressure and volume of
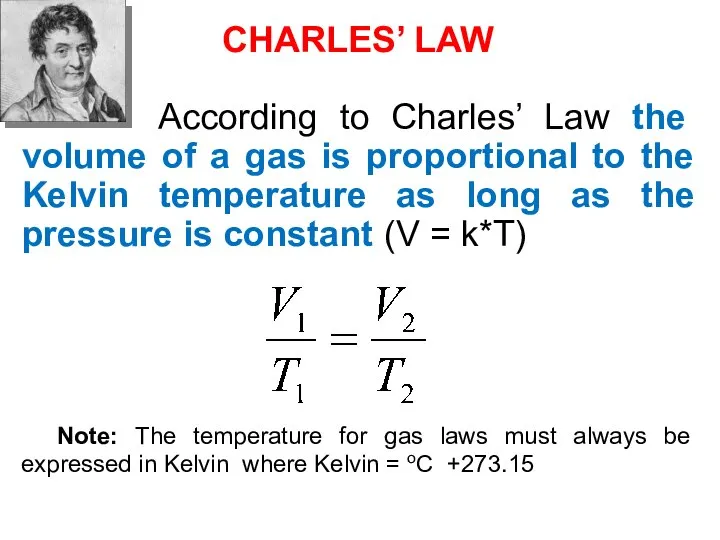
- 40. CHARLES’ LAW According to Charles’ Law the volume of a gas is proportional to the Kelvin
- 41. GAY-LUSSAC'S LAW Gay Lussac's Law of pressure and temperature describes the direct relationship between pressure and
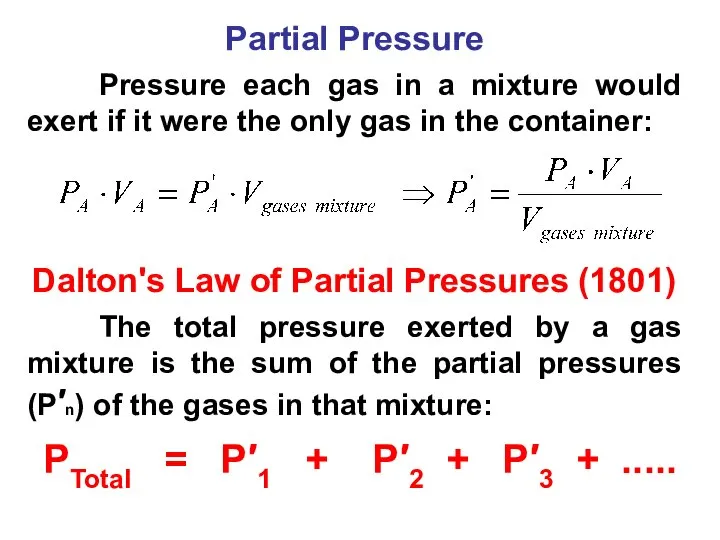
- 42. Partial Pressure Pressure each gas in a mixture would exert if it were the only gas
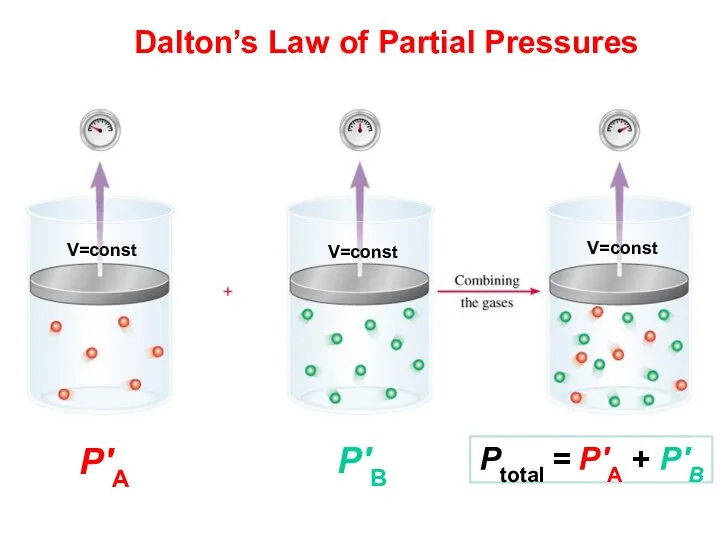
- 43. Dalton’s Law of Partial Pressures Ptotal = P′А + P′В P′В P′А V=const V=const V=const
- 45. Скачать презентацию



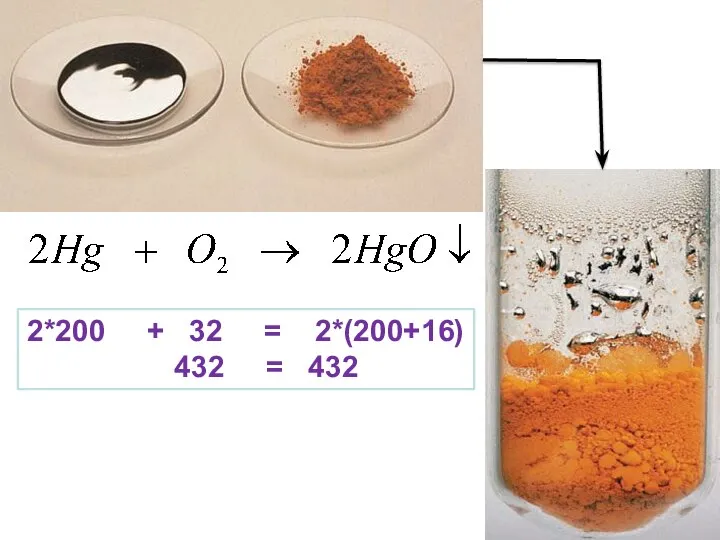



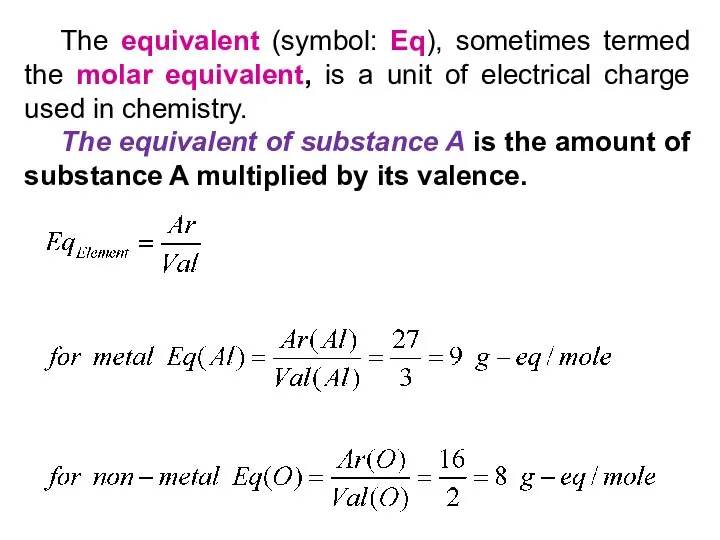
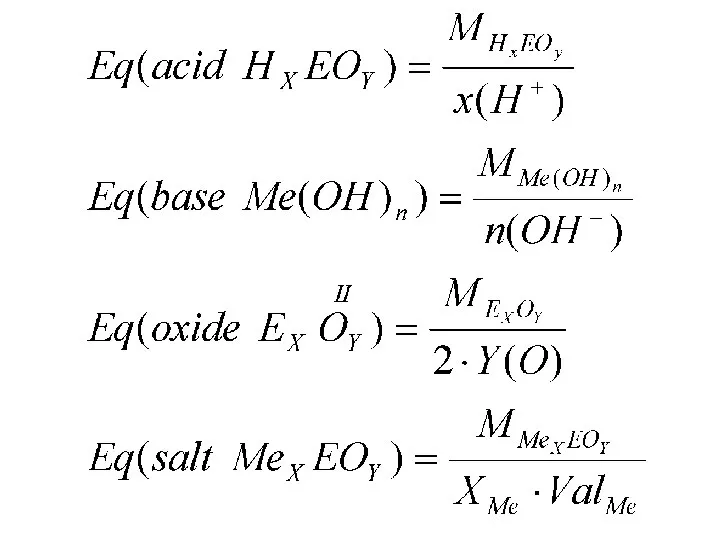


 Тесты по неорганической химии
Тесты по неорганической химии Автор презентации: Короткова Екатерина Викторовна, учитель биологии и химии МОУ «Горютинская СОШ» Биология 9 класс
Автор презентации: Короткова Екатерина Викторовна, учитель биологии и химии МОУ «Горютинская СОШ» Биология 9 класс Классификация химических реакций Маланина Елена Алексеевна Учитель химии МОУ Большевяземская гимназия, р.п. Большие Вяземы,
Классификация химических реакций Маланина Елена Алексеевна Учитель химии МОУ Большевяземская гимназия, р.п. Большие Вяземы,  Карбоновые кислоты
Карбоновые кислоты Сверхразветвленные полимеры: типы, получение, применение
Сверхразветвленные полимеры: типы, получение, применение Хлоропласты. Митохондрии
Хлоропласты. Митохондрии V група періодичної системи Менделєєва
V група періодичної системи Менделєєва Расчетная ячейка при МД моделировании. Граничные условия. Элементарная ячейка для атомов аргона
Расчетная ячейка при МД моделировании. Граничные условия. Элементарная ячейка для атомов аргона Состояние электронов в атоме
Состояние электронов в атоме Значение воды. Роль водного фактора в формировании здоровья населения
Значение воды. Роль водного фактора в формировании здоровья населения Презентация по Химии "Кристалл душы" - скачать смотреть
Презентация по Химии "Кристалл душы" - скачать смотреть  Презентация по Химии "Фосфор" - скачать смотреть бесплатно_
Презентация по Химии "Фосфор" - скачать смотреть бесплатно_ Кальций(Ca)
Кальций(Ca) Нафта, вугілля, природний газ як вуглеводнева сировина Підготувала учениця 11 класу Дерев’янко Ірина
Нафта, вугілля, природний газ як вуглеводнева сировина Підготувала учениця 11 класу Дерев’янко Ірина  Информационные технологии в обучении химии
Информационные технологии в обучении химии Углеводы
Углеводы Тема : Спирты Презентация по химии Ученицы 11 «Б» класса ЗОШ №41 Зануды Татьяны
Тема : Спирты Презентация по химии Ученицы 11 «Б» класса ЗОШ №41 Зануды Татьяны  Факторы, влияющие на скорость химической реакции
Факторы, влияющие на скорость химической реакции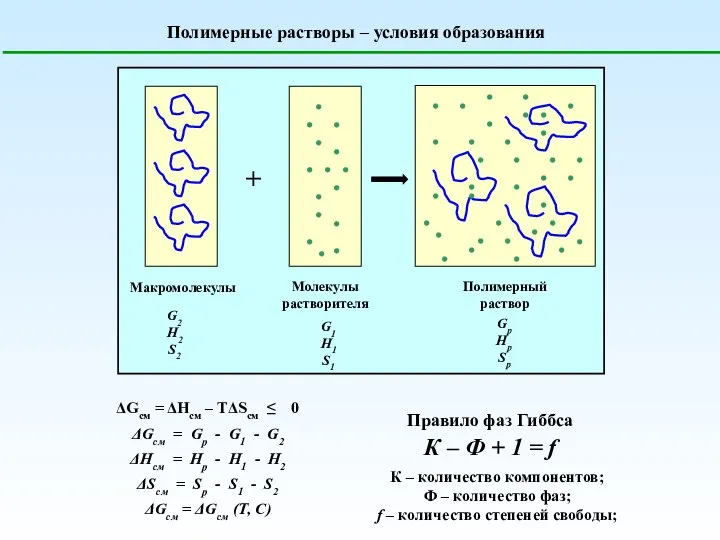 Полимерные растворы – условия образования
Полимерные растворы – условия образования Коррозия металлов
Коррозия металлов «ШАРИКИ СЧАСТЬЯ» Неустроева Светлана Габдульхатовна, учитель I категории, МБОУ «СОШ №31», г.Нижнекамск
«ШАРИКИ СЧАСТЬЯ» Неустроева Светлана Габдульхатовна, учитель I категории, МБОУ «СОШ №31», г.Нижнекамск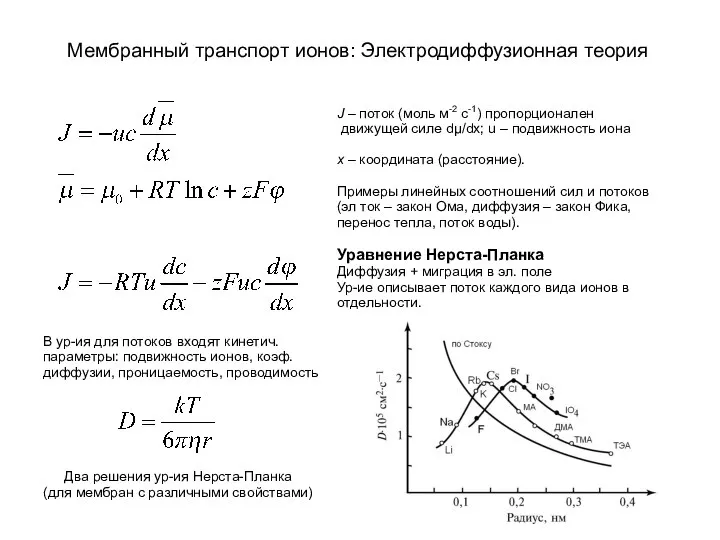 Мембранный транспорт ионов: электродиффузионная теория
Мембранный транспорт ионов: электродиффузионная теория Химико-фармацевтические препараты
Химико-фармацевтические препараты Презентация по химии Олигосахариды ПОЛИСАХАРИДЫ.
Презентация по химии Олигосахариды ПОЛИСАХАРИДЫ. 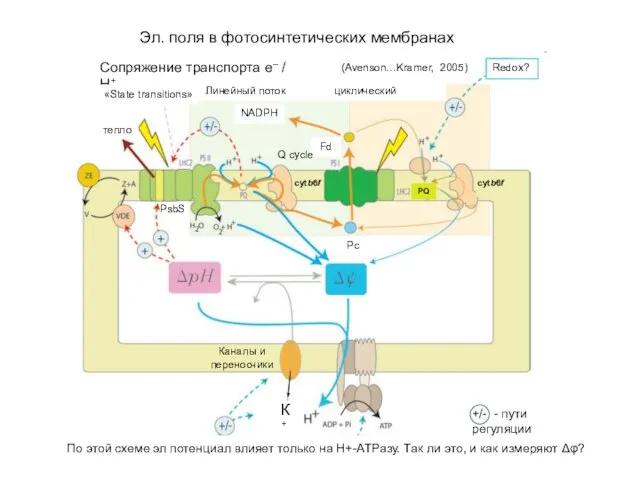 Электрические поля в фотосинтетических мембранах
Электрические поля в фотосинтетических мембранах Введение в термические процессы вторичной переработки нефти
Введение в термические процессы вторичной переработки нефти Закономірності протікання хімічних реакцій
Закономірності протікання хімічних реакцій Термореактивные и термопластичные пластмассы
Термореактивные и термопластичные пластмассы