Содержание
- 2. PLAN Classification of carbohydrates. Nomenclature. Structural representations be Fisher and Haworth. Chirality. Optical isomers. Tautomerism. Mutarotation.
- 3. Carbohydrates The term "carbohydrate" was proposed by K.G. Shmidt in 1844. Cn(H2O)m (n=3-9) A carbohydrate is
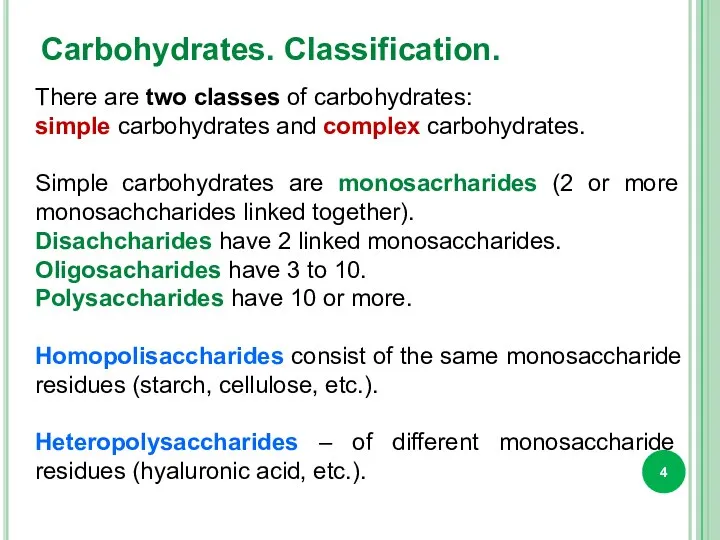
- 4. Carbohydrates. Classification. There are two classes of carbohydrates: simple carbohydrates and complex carbohydrates. Simple carbohydrates are
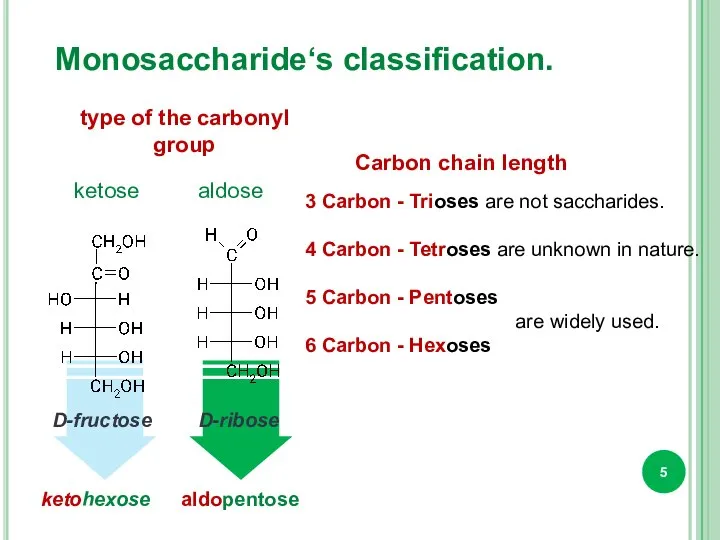
- 5. Monosaccharide‘s classification. type of the carbonyl group Carbon chain length 3 Carbon - Trioses are not
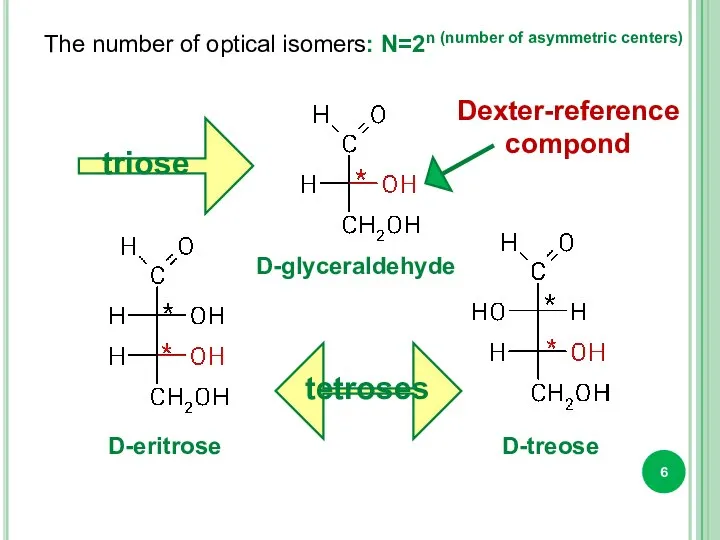
- 6. The number of optical isomers: N=2n (number of asymmetric centers) triose tetroses D-glyceraldehyde D-eritrose D-treose Dexter-reference
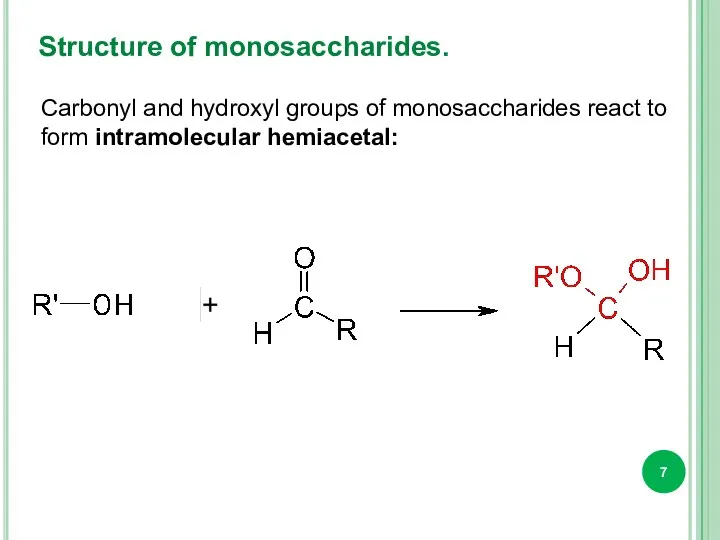
- 7. Structure of monosaccharides. Carbonyl and hydroxyl groups of monosaccharides react to form intramolecular hemiacetal:
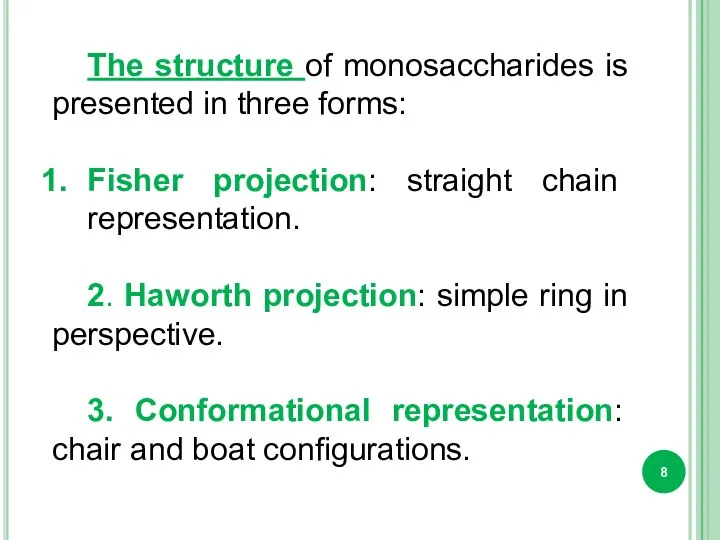
- 8. The structure of monosaccharides is presented in three forms: Fisher projection: straight chain representation. 2. Haworth
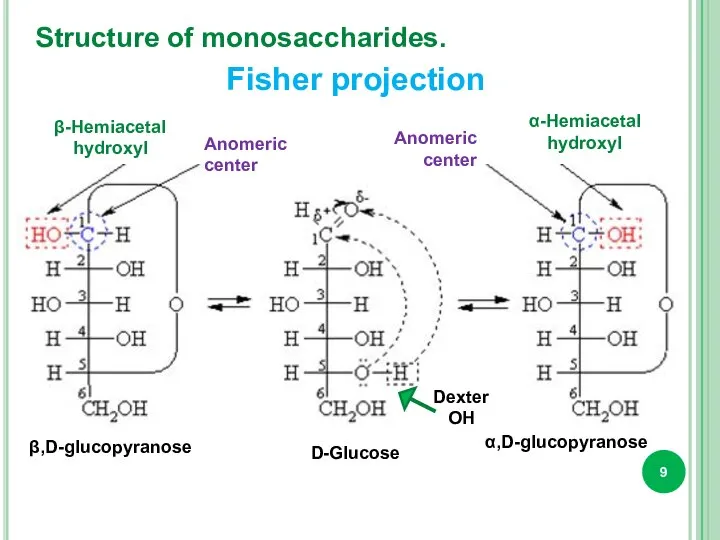
- 9. Structure of monosaccharides. β-Hemiacetal hydroxyl α-Hemiacetal hydroxyl Anomeric center Anomeric center D-Glucose β,D-glucopyranose α,D-glucopyranose Dexter OH
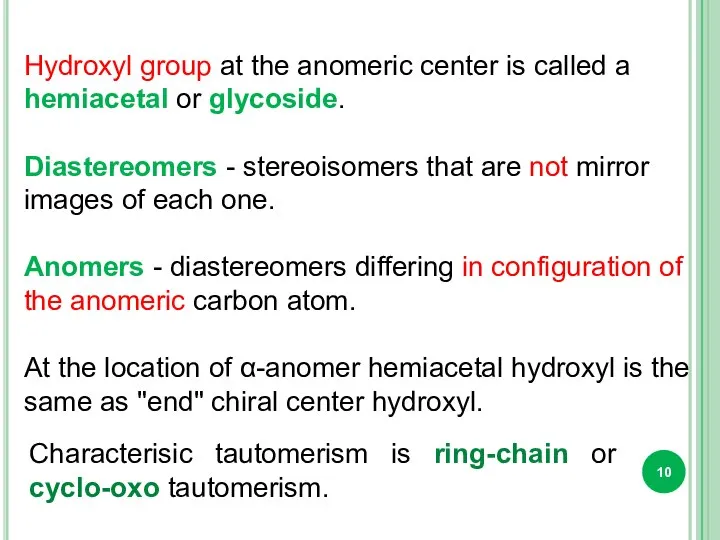
- 10. Hydroxyl group at the anomeric center is called a hemiacetal or glycoside. Diastereomers - stereoisomers that
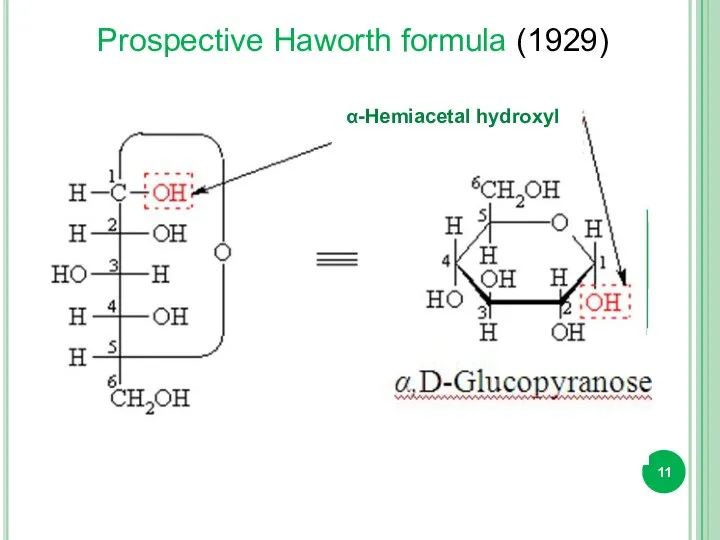
- 11. α-Hemiacetal hydroxyl Prospective Haworth formula (1929)
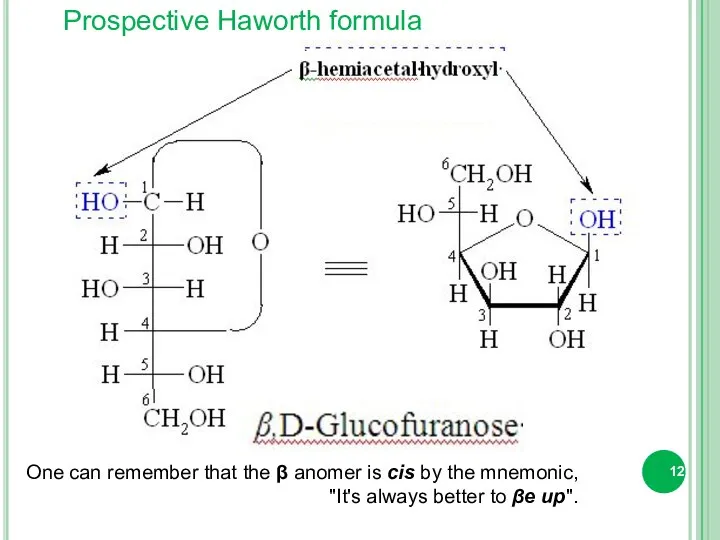
- 12. Prospective Haworth formula One can remember that the β anomer is cis by the mnemonic, "It's
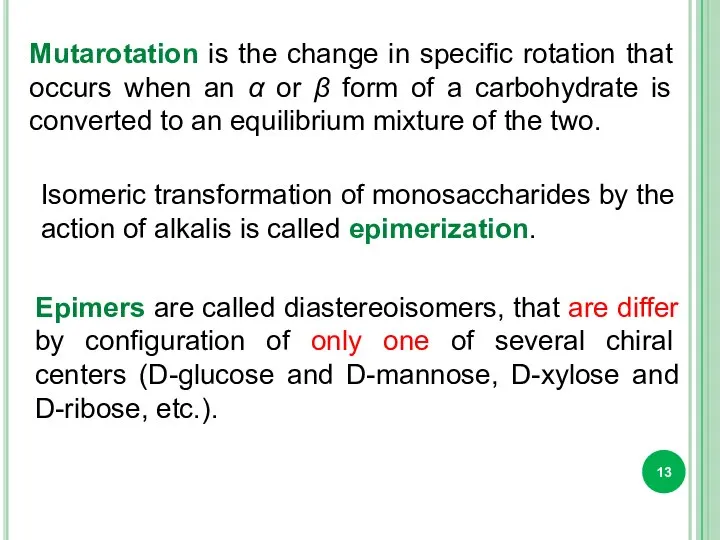
- 13. Isomeric transformation of monosaccharides by the action of alkalis is called epimerization. Epimers are called diastereoisomers,
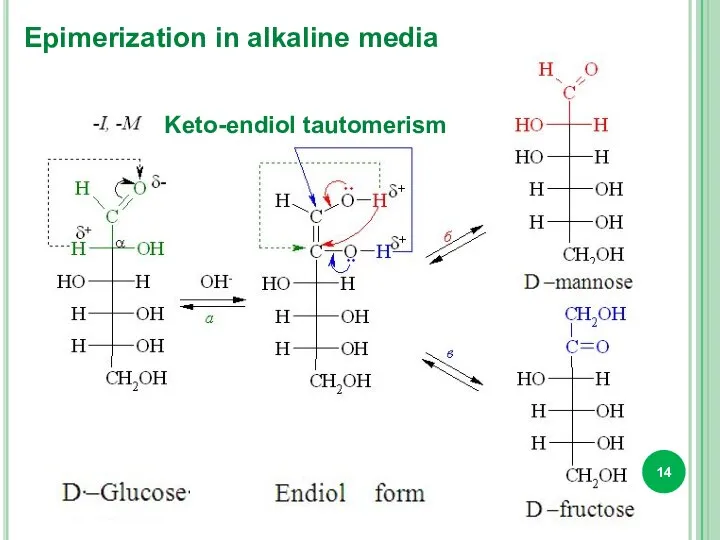
- 14. Keto-endiol tautomerism Epimerization in alkaline media
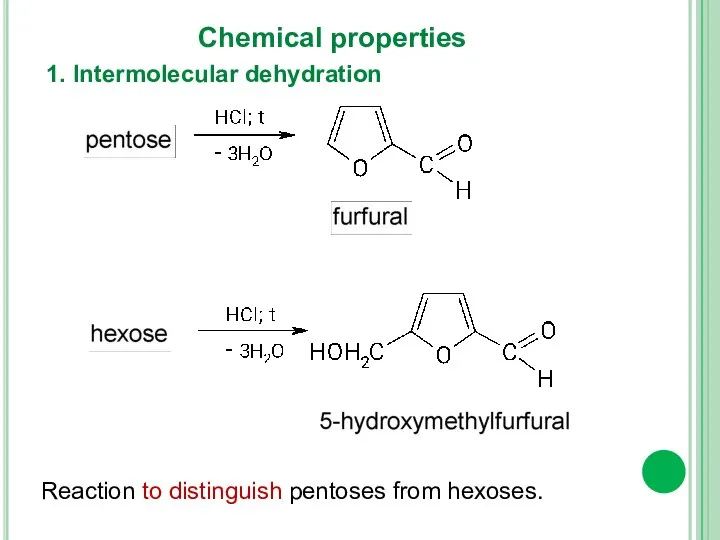
- 15. 1. Intermolecular dehydration Chemical properties Reaction to distinguish pentoses from hexoses.
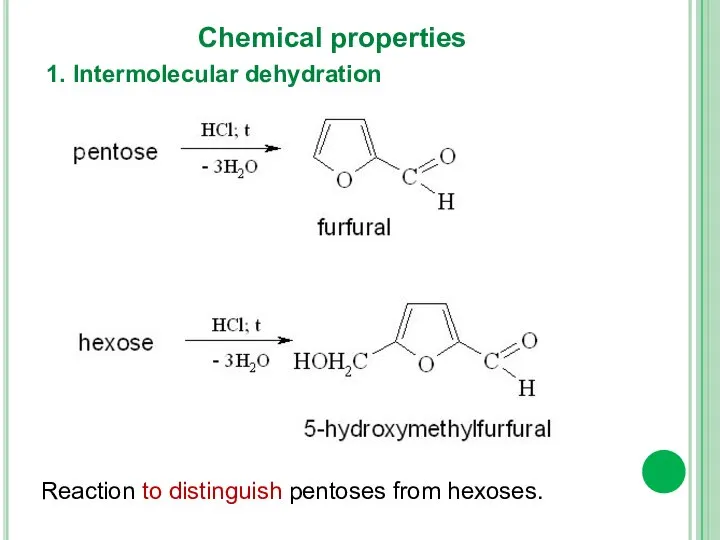
- 16. 1. Intermolecular dehydration Chemical properties Reaction to distinguish pentoses from hexoses.
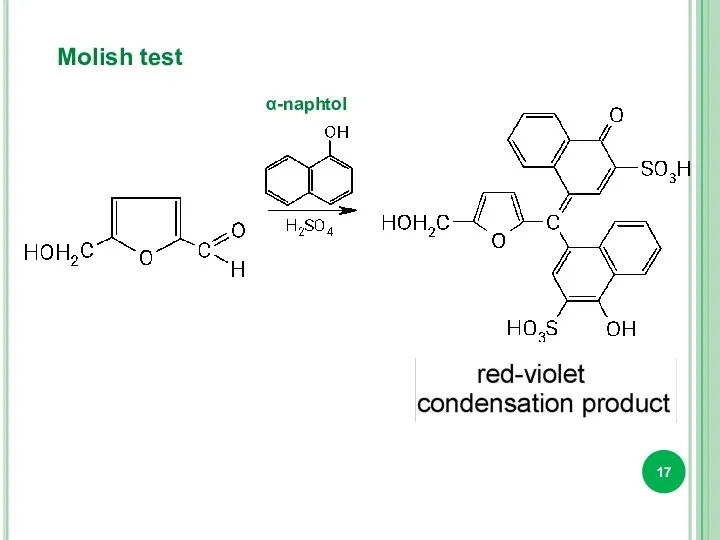
- 17. Molish test α-naphtol
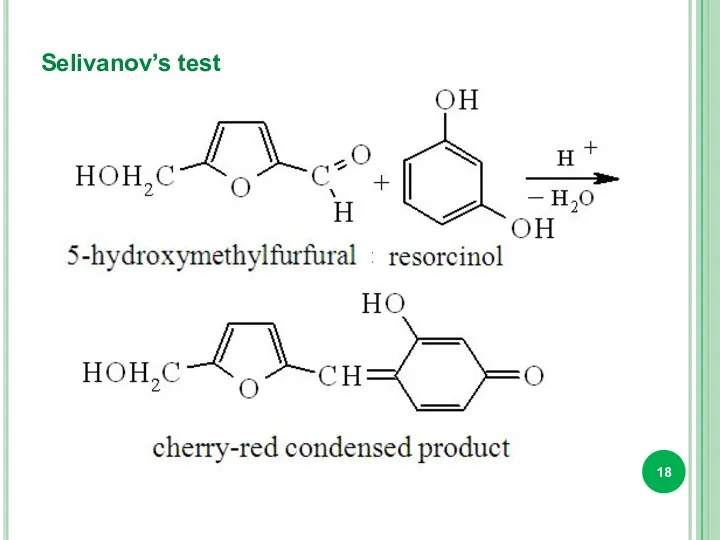
- 18. Selivanov’s test
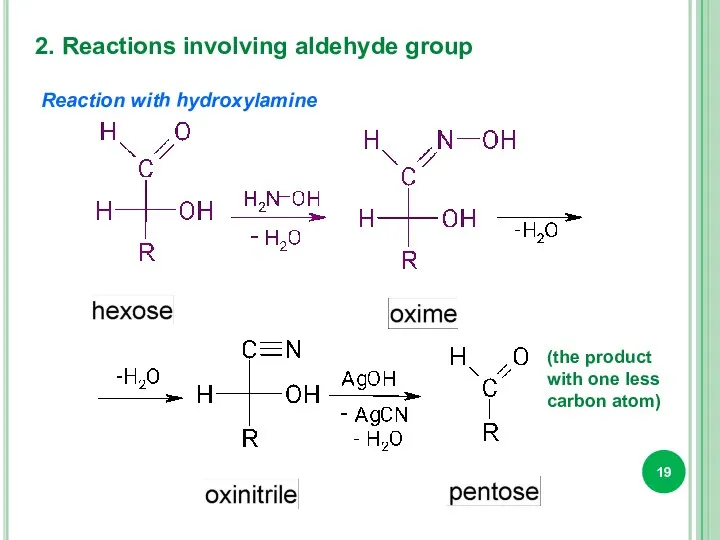
- 19. 2. Reactions involving aldehyde group Reaction with hydroxylamine (the product with one less carbon atom)
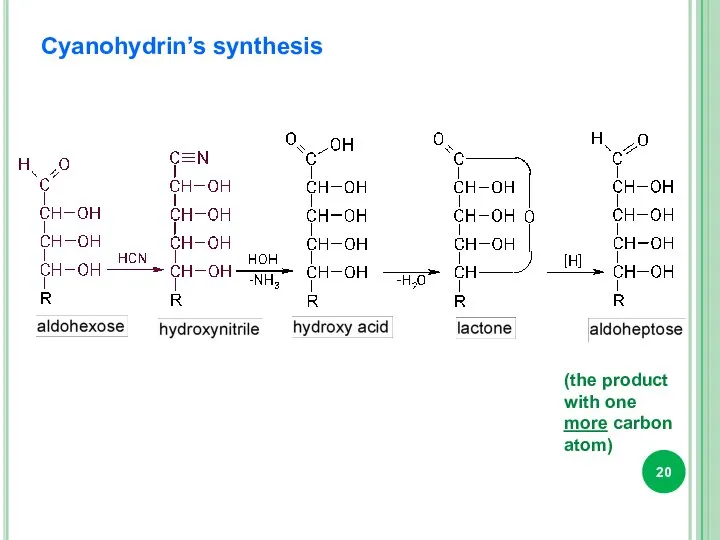
- 20. Cyanohydrin’s synthesis (the product with one more carbon atom)
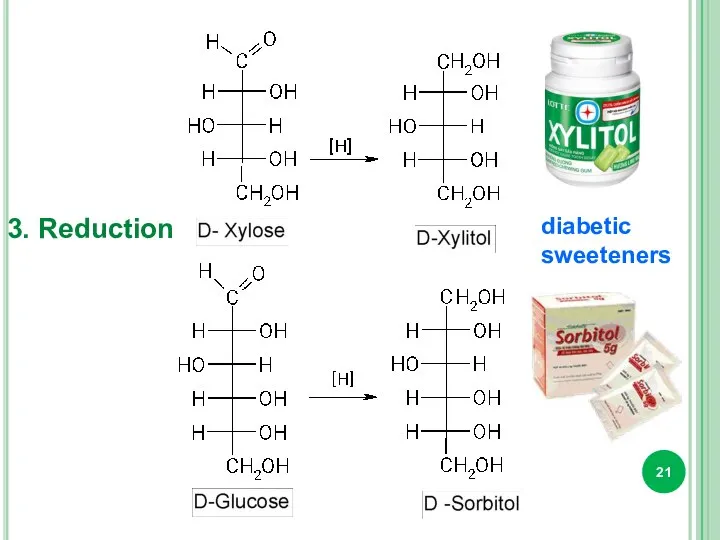
- 21. 3. Reduction diabetic sweeteners
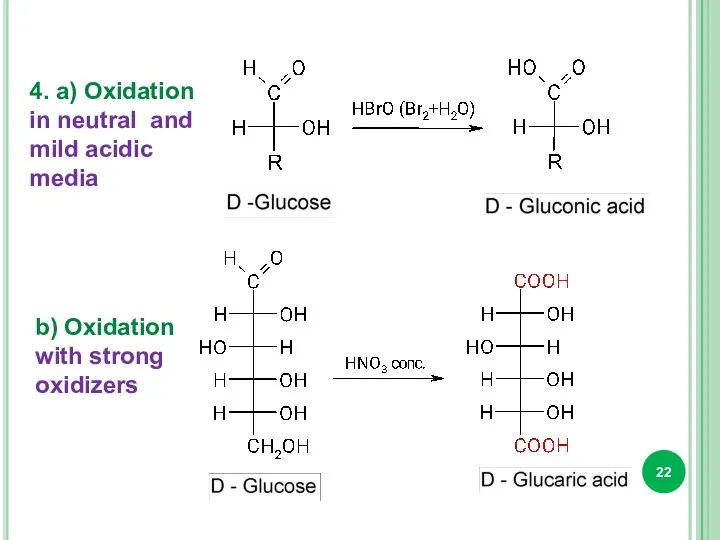
- 22. 4. a) Oxidation in neutral and mild acidic media b) Oxidation with strong oxidizers
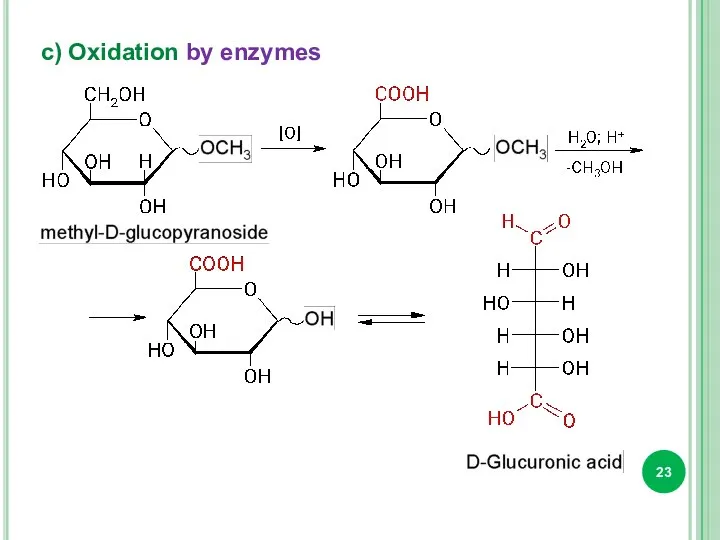
- 23. c) Oxidation by enzymes
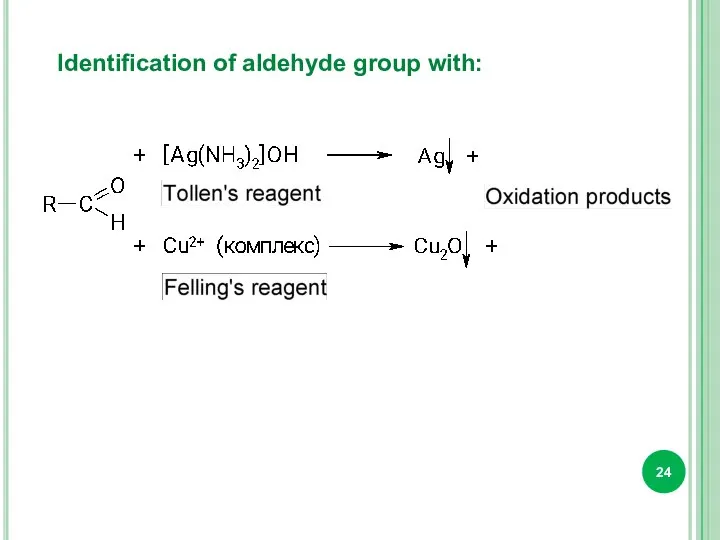
- 24. Identification of aldehyde group with:
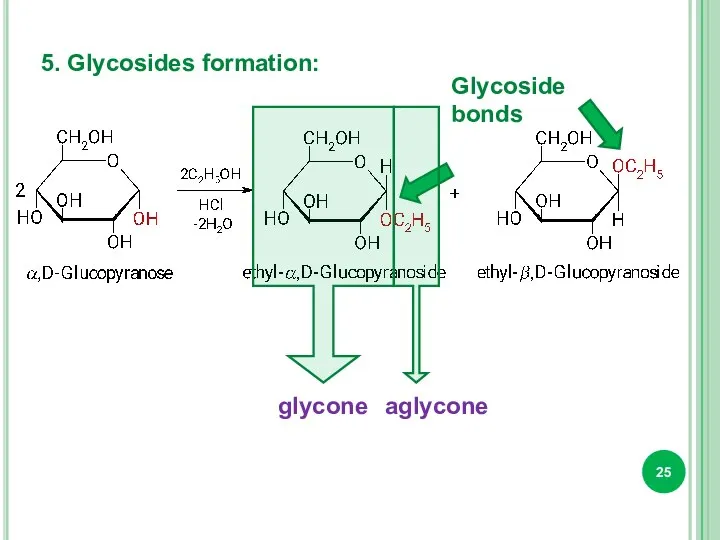
- 25. 5. Glycosides formation: Glycoside bonds glycone aglycone
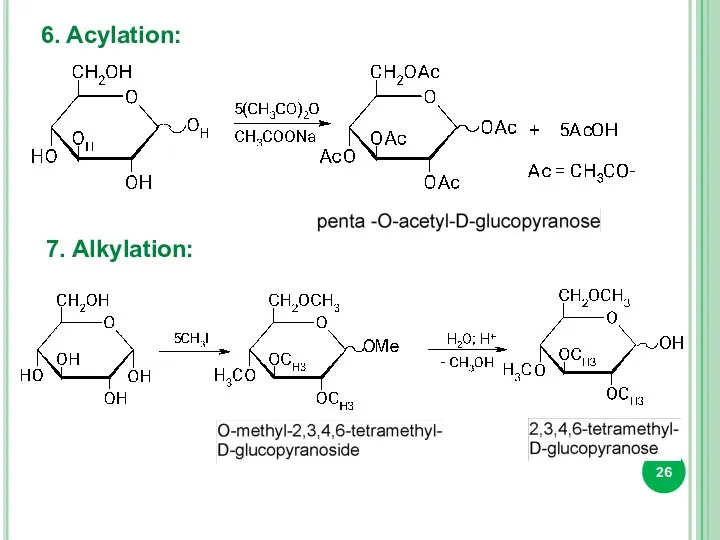
- 26. 6. Acylation: 7. Alkylation:
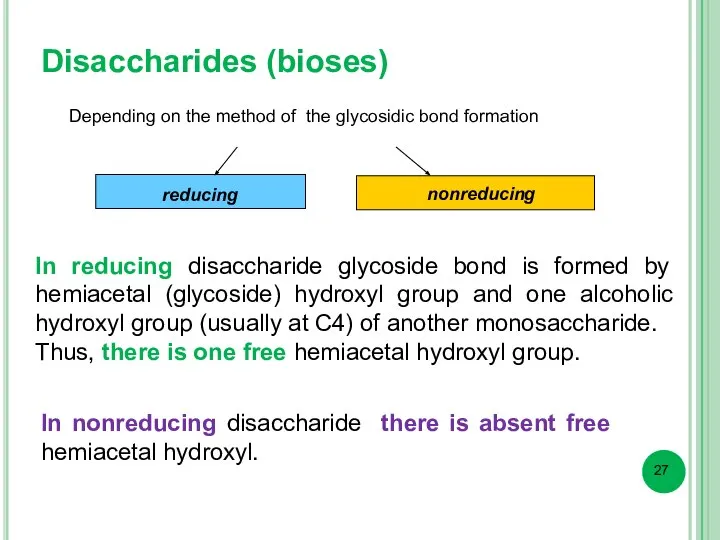
- 27. Disaccharides (bioses) Depending on the method of the glycosidic bond formation reducing nonreducing In reducing disaccharide
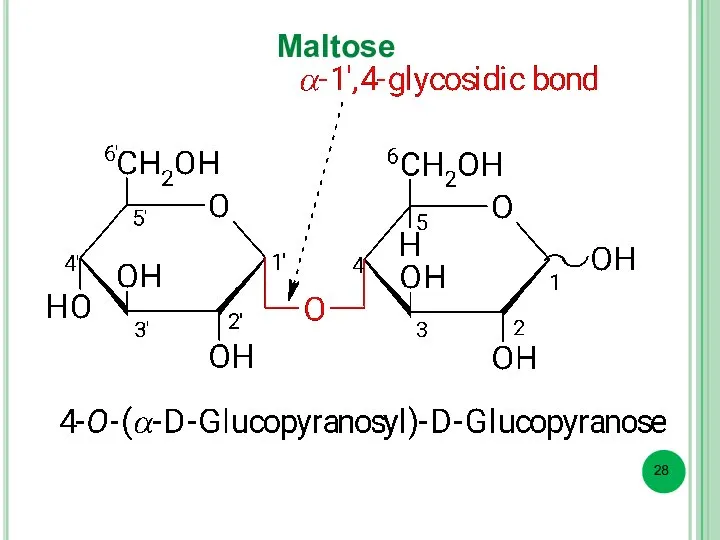
- 28. Maltose
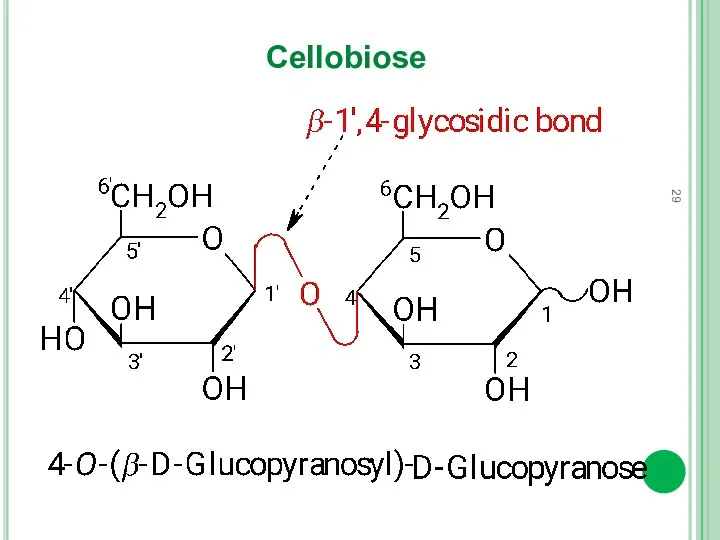
- 29. Cellobiose
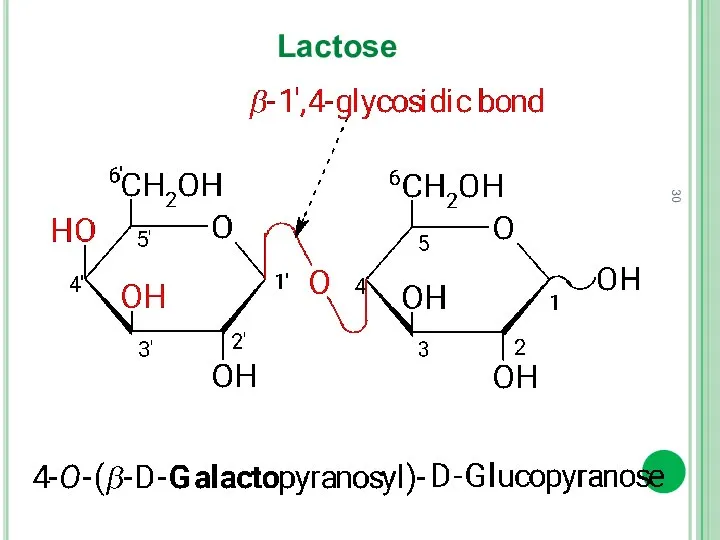
- 30. Lactose
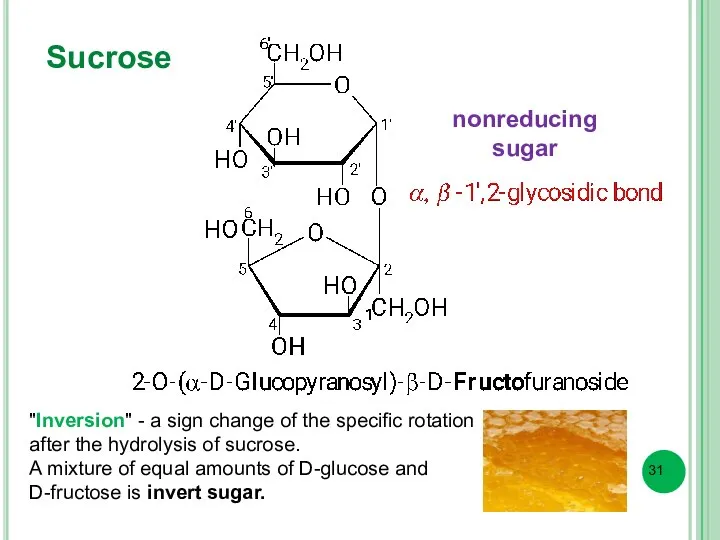
- 31. Sucrose "Inversion" - a sign change of the specific rotation after the hydrolysis of sucrose. A
- 32. Sucrose. chemical properties. Doesn’t mutorotate No silver mirror reaction No reactions by aldehyde group Hydrolysing to
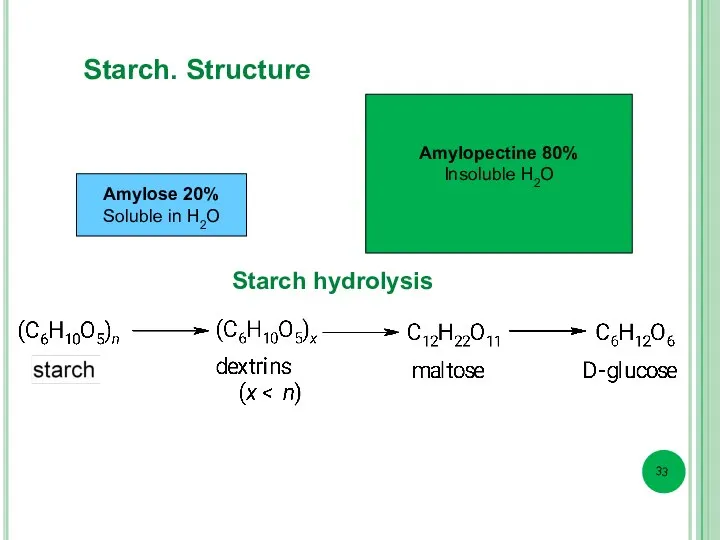
- 33. Amylose 20% Soluble in Н2О Amylopectine 80% Insoluble Н2О Starch hydrolysis Starch. Structure
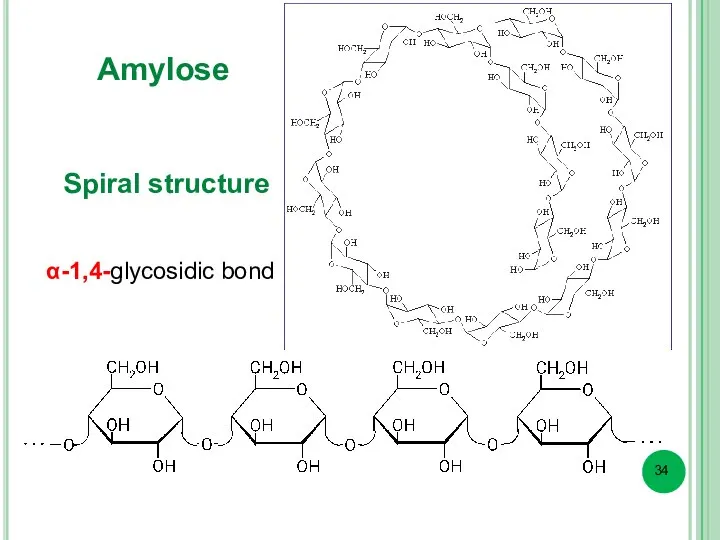
- 34. Amylose α-1,4-glycosidic bond Spiral structure
- 35. Amylose with iodine forms clastrates of dark blue color.
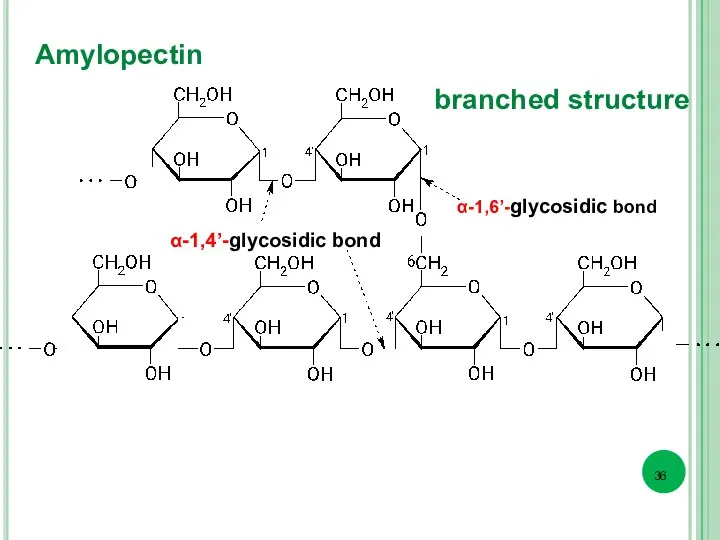
- 36. Amylopectin α-1,6’-glycosidic bond α-1,4’-glycosidic bond branched structure
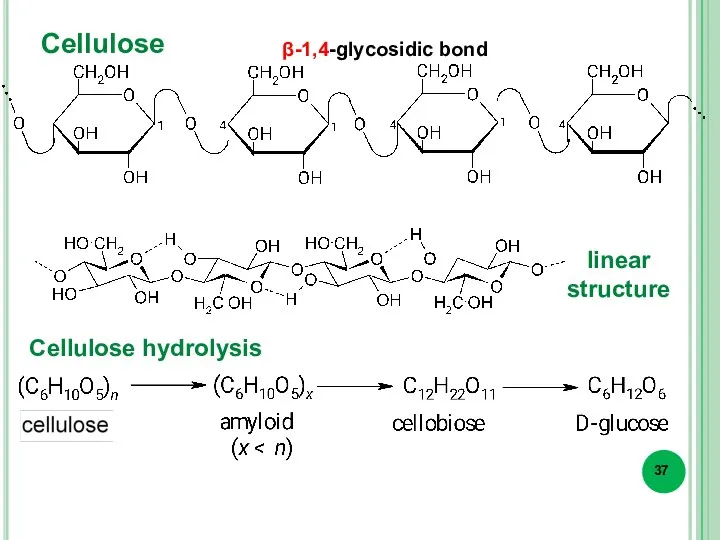
- 37. Cellulose Cellulose hydrolysis β-1,4-glycosidic bond linear structure
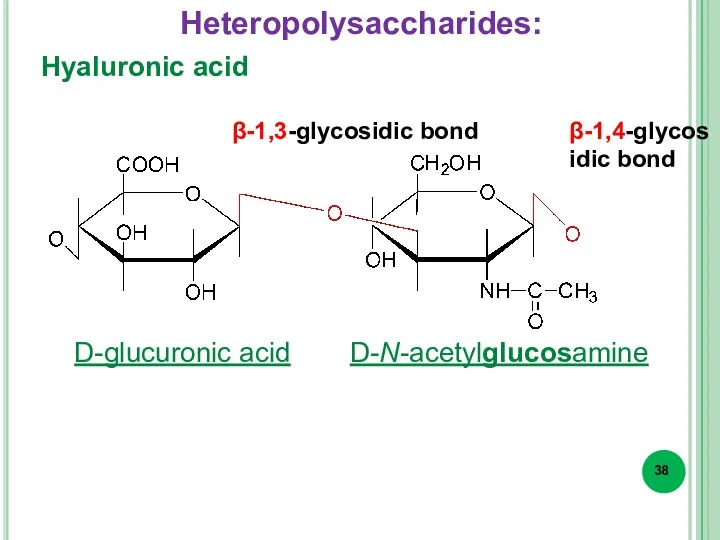
- 38. Hyaluronic acid β-1,3-glycosidic bond β-1,4-glycosidic bond D-glucuronic acid D-N-acetylglucosamine Heteropolysaccharides:
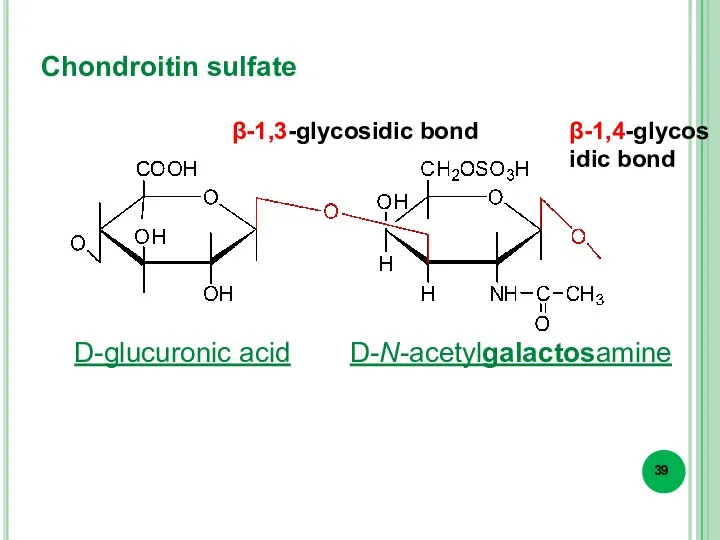
- 39. Chondroitin sulfate β-1,3-glycosidic bond β-1,4-glycosidic bond D-glucuronic acid D-N-acetylgalactosamine
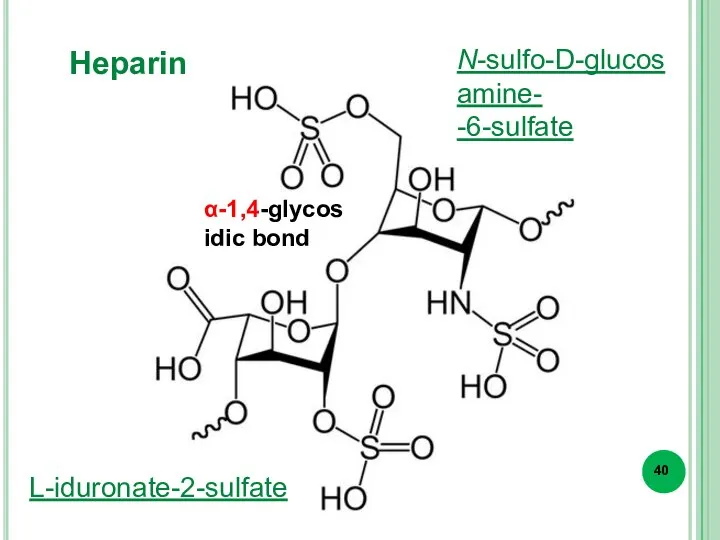
- 40. Heparin L-iduronate-2-sulfate N-sulfo-D-glucosamine- -6-sulfate α-1,4-glycosidic bond
- 42. Скачать презентацию



 Физические и химические свойства меди
Физические и химические свойства меди Решаем задачи «Домашняя аптечка» Карпухина Ирина Степановна Учитель химии МБОУ СОШ № 32 Город Новосибирск
Решаем задачи «Домашняя аптечка» Карпухина Ирина Степановна Учитель химии МБОУ СОШ № 32 Город Новосибирск Природный и синтетический каучуки. Резина
Природный и синтетический каучуки. Резина Энергетика химических процессов. Основы термохимии
Энергетика химических процессов. Основы термохимии Аттестационная работа. Организация деятельности по изучению природных и искусственных красителей для пасхальных яиц
Аттестационная работа. Организация деятельности по изучению природных и искусственных красителей для пасхальных яиц Альдегиды
Альдегиды Неорганическая химия Сероводород
Неорганическая химия Сероводород Химико – математические проценты
Химико – математические проценты Оценка химической обстановки при авариях на химически опасных объектах
Оценка химической обстановки при авариях на химически опасных объектах Знаки химических элементов. Дополнение
Знаки химических элементов. Дополнение Диффузия золота и свинца
Диффузия золота и свинца Минеральные кислоты
Минеральные кислоты Хімічний склад і використання мінералів
Хімічний склад і використання мінералів Строение атома
Строение атома Геохимия природных процессов
Геохимия природных процессов Пәннiң мiндетi мен мақсаты. Термодинамика. Термохимия
Пәннiң мiндетi мен мақсаты. Термодинамика. Термохимия Биологическая коррозия и защита строительных материалов и конструкций от биоповреждений
Биологическая коррозия и защита строительных материалов и конструкций от биоповреждений Презентация по Химии "Хлор" - скачать смотреть
Презентация по Химии "Хлор" - скачать смотреть  Алканы. Практическое занятие 1
Алканы. Практическое занятие 1 Бытовая химия в нашей жизни
Бытовая химия в нашей жизни Драгоценные камни
Драгоценные камни Кислоты. Соли (8 класс)
Кислоты. Соли (8 класс) Уравнение состояния идеального газа
Уравнение состояния идеального газа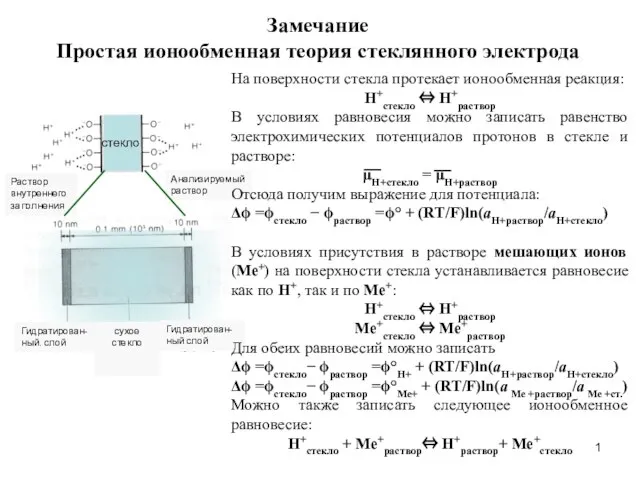 Простая ионообменная теория стеклянного электрода
Простая ионообменная теория стеклянного электрода Центрифугирование в почвоведении
Центрифугирование в почвоведении Расчеты равновесий в водных растворах рН, рОН
Расчеты равновесий в водных растворах рН, рОН Бейорганикалық қосылыстардың негізгі кластары
Бейорганикалық қосылыстардың негізгі кластары S,p,d,f-элементтері және олардың биологиялық маңызы
S,p,d,f-элементтері және олардың биологиялық маңызы