Содержание
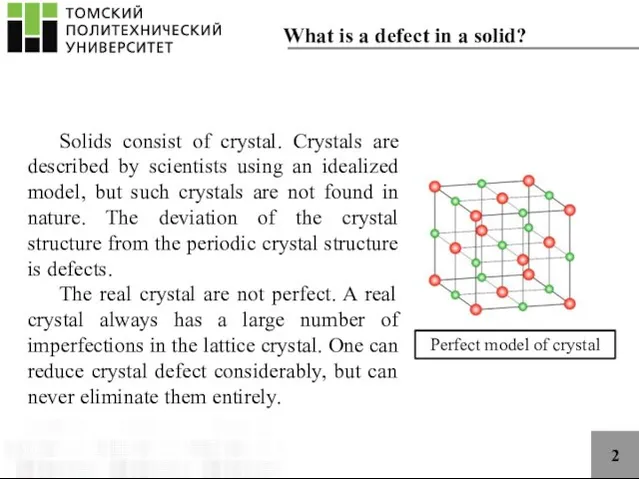
- 2. 2 Solids consist of crystal. Crystals are described by scientists using an idealized model, but such
- 3. 3 Dictionary Solid – твердое тело, твердое состояние Sufficiently – достаточно Impurities – примеси Invariably –
- 4. 4 Defects occur in a solid at any temperature. The number of defects increases with increasing
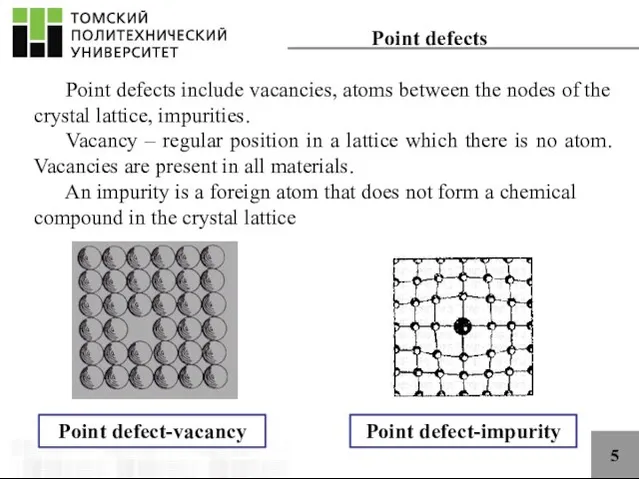
- 5. 5 Point defects Point defects include vacancies, atoms between the nodes of the crystal lattice, impurities.
- 6. 6 Defect of Schotky and Frenkel Frenkel defect: anion vacancy-interstitial cation pair When the temperature is
- 7. 7 Number of defect of Schotky The number of vacancies (defect of Shotky) is given by
- 8. 8 Number of defect of Frenkel The number of defect of pair Frenkel is given by
- 9. Line defect - dislocation The experimental data do not show that the observed values of the
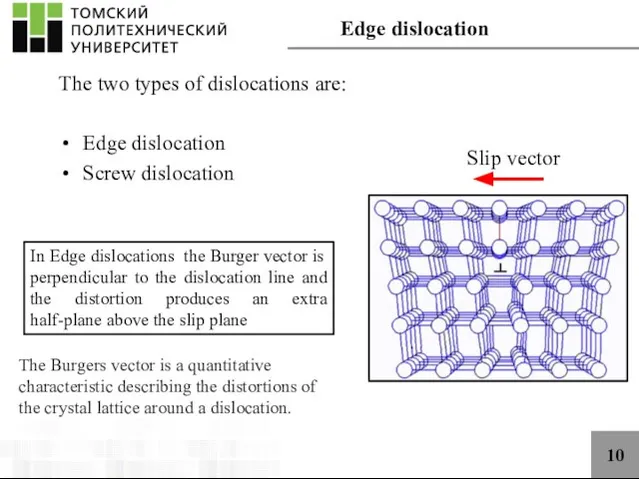
- 10. 10 The two types of dislocations are: Edge dislocation Screw dislocation In Edge dislocations the Burger
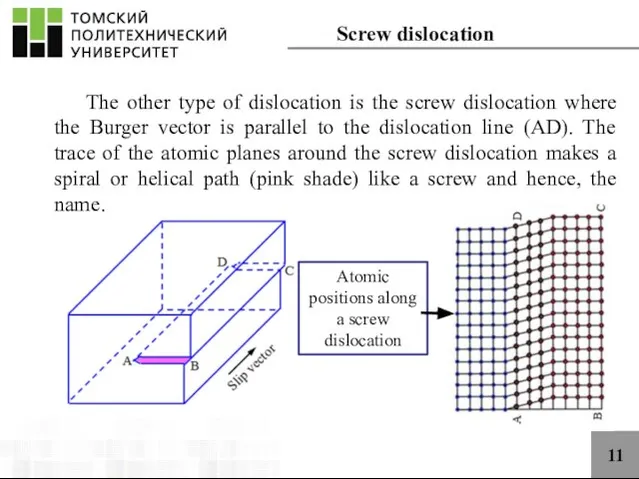
- 11. 11 The other type of dislocation is the screw dislocation where the Burger vector is parallel
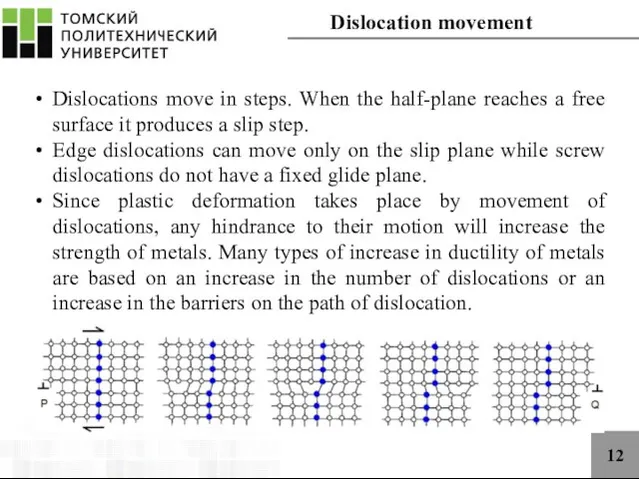
- 12. 12 Dislocations move in steps. When the half-plane reaches a free surface it produces a slip
- 13. 13 Dislocations appear as lines when observed under transmission electron microscope (TEM) Observing dislocations
- 14. 14 Grain boundaries is surface defect. Most crystalline solids are an aggregate of several crystals. Such
- 15. 15 Porosity Cracks Inclusions These defects form during manufacturing processes for various reasons and often harmful
- 16. 16 Volume defect Microphotograph of steel with inclusions of enriched Uranium
- 17. 17 Volume defect Another example is effect Hydrogen Embrittlement. It is generally agreed that hydrogen, in
- 18. 18 The best known methods for studying defects in solid: Measurement of electrical resistance Positron annihilation
- 20. Скачать презентацию










 Можно ли выкрасить кристаллы в домашних условиях
Можно ли выкрасить кристаллы в домашних условиях Качественные реакции
Качественные реакции Аммиак
Аммиак Комплексные соединения
Комплексные соединения Введение. Основные понятия химии
Введение. Основные понятия химии Ксенобиотики. Микросомальное окисление
Ксенобиотики. Микросомальное окисление Молекулярно-кінетичні явища в дисперсних системах
Молекулярно-кінетичні явища в дисперсних системах Имидазол. Получение, свойства, анализ, применение, условия хранения лекарственных препаратов производных имидазола
Имидазол. Получение, свойства, анализ, применение, условия хранения лекарственных препаратов производных имидазола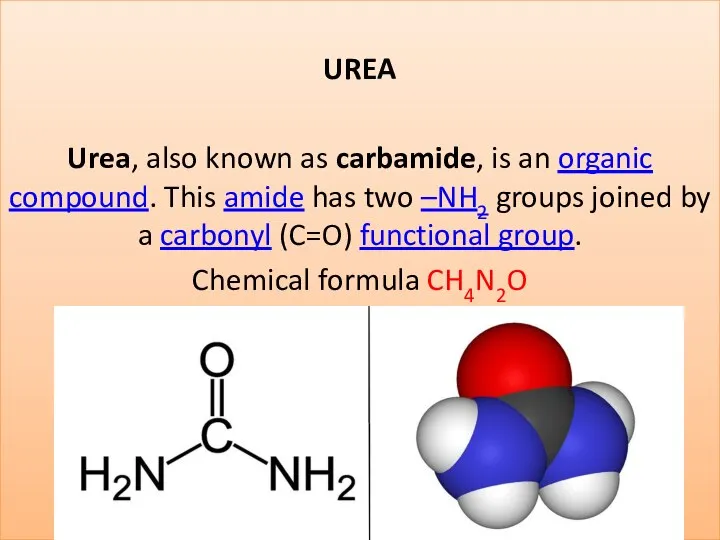 Urea (carbamide)
Urea (carbamide) Презентация по Химии "Силікатні матеріали: скло, кераміка, цемент" - скачать смотреть бесплатно
Презентация по Химии "Силікатні матеріали: скло, кераміка, цемент" - скачать смотреть бесплатно МОЛЯРНЫЙ ОБЪЕМ Химия 8 класс
МОЛЯРНЫЙ ОБЪЕМ Химия 8 класс Хімічний склад і використання мінералів
Хімічний склад і використання мінералів Реакционная способность карбоновых кислот
Реакционная способность карбоновых кислот Современное состояние и пути совершенствования стандартизации лекарственных средств
Современное состояние и пути совершенствования стандартизации лекарственных средств Номенклатура органических соединений
Номенклатура органических соединений Электрохимическая коррозии. Катодные процессы электрохимической коррозии
Электрохимическая коррозии. Катодные процессы электрохимической коррозии Экспериментальное исследование кипения сверхтекучего гелия на цилиндрическом нагревателе внутри пористой оболочки
Экспериментальное исследование кипения сверхтекучего гелия на цилиндрическом нагревателе внутри пористой оболочки Альдегиды. Строение молекул
Альдегиды. Строение молекул Фосфор. Соединения фосфора
Фосфор. Соединения фосфора Углеводороды. Алканы
Углеводороды. Алканы Биотит и тальк
Биотит и тальк Органикалық қосылыстар
Органикалық қосылыстар Практическая работа № 3. Получение, собирание и распознавание газов
Практическая работа № 3. Получение, собирание и распознавание газов Презентация по Химии "Вода" - скачать смотреть
Презентация по Химии "Вода" - скачать смотреть  Качественный и количественный методы исследования растворов
Качественный и количественный методы исследования растворов ГСМ - горюче-смазочные материалы
ГСМ - горюче-смазочные материалы Сероводород
Сероводород Пищевые добавки
Пищевые добавки