Содержание
- 2. Today’s objectives: Define electrode, anode, cathode, anion, cation, salt bridge/porous cup, electrolyte, and voltaic cell Predict
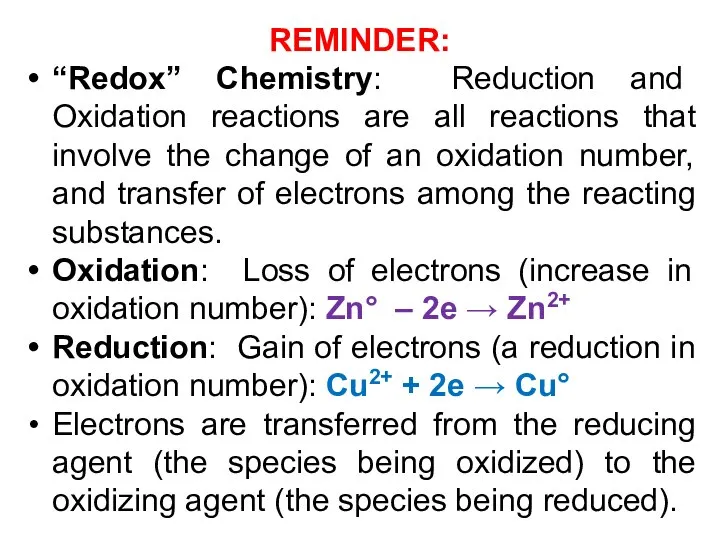
- 3. REMINDER: “Redox” Chemistry: Reduction and Oxidation reactions are all reactions that involve the change of an
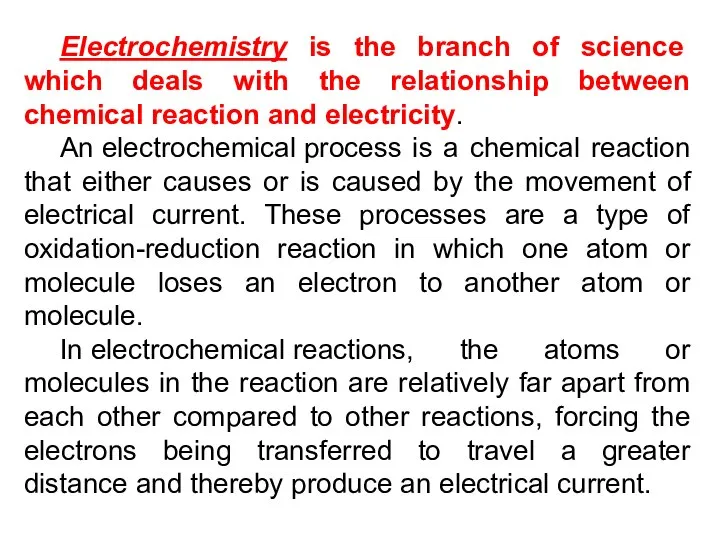
- 4. Electrochemistry is the branch of science which deals with the relationship between chemical reaction and electricity.
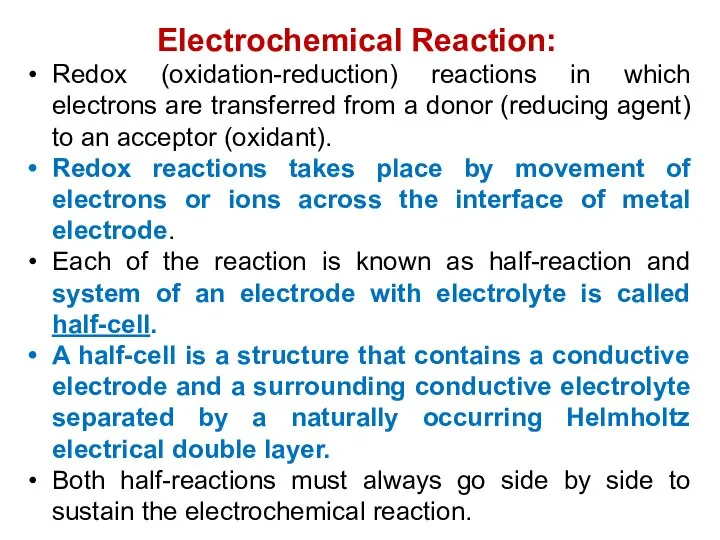
- 5. Electrochemical Reaction: Redox (oxidation-reduction) reactions in which electrons are transferred from a donor (reducing agent) to
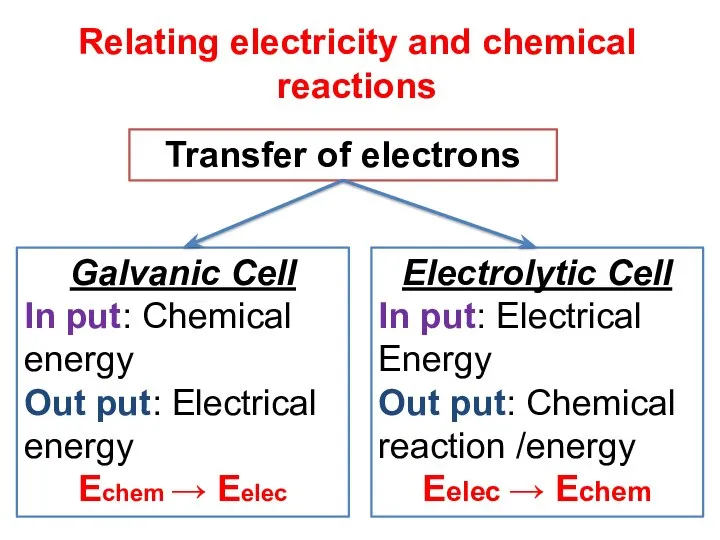
- 6. Relating electricity and chemical reactions Transfer of electrons Galvanic Cell In put: Chemical energy Out put:
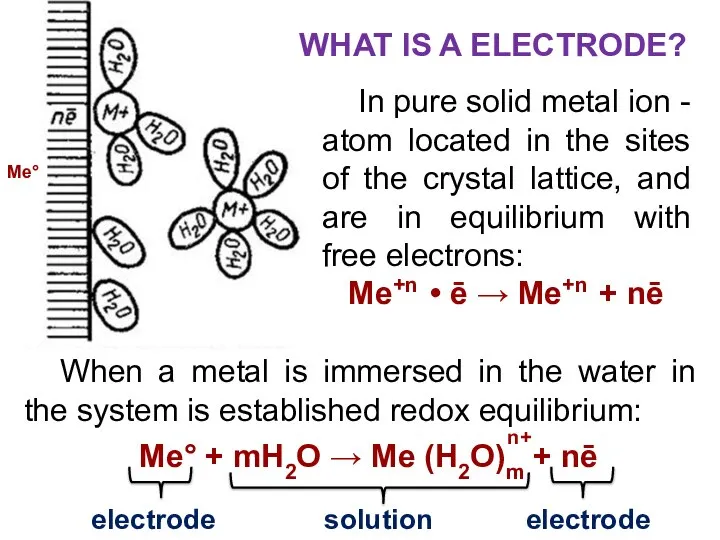
- 7. In pure solid metal ion - atom located in the sites of the crystal lattice, and
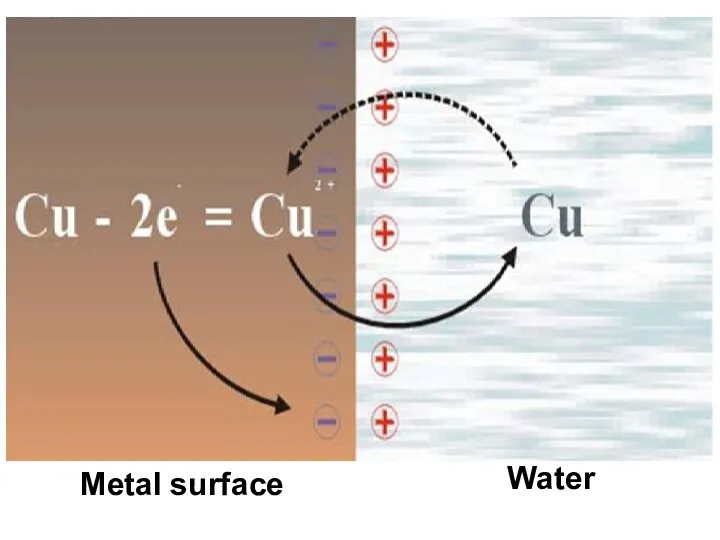
- 8. Metal surface Water
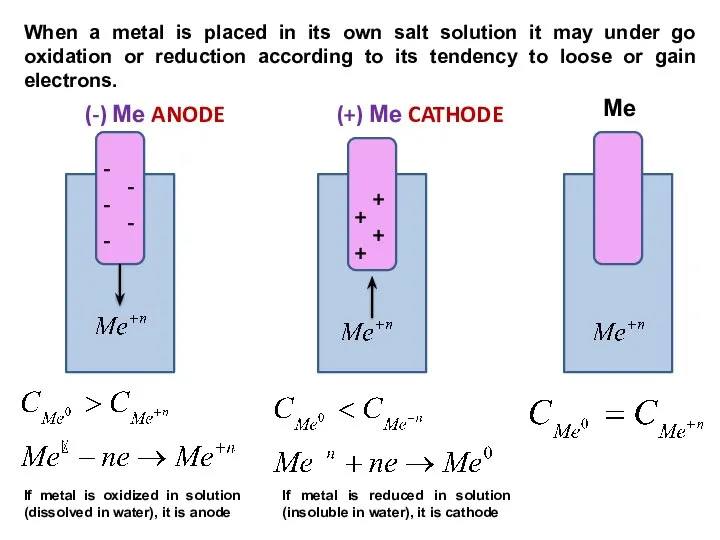
- 9. (-) Ме ANODE (+) Ме CATHODE Ме - - - - - + + + +
- 10. An electrode in an electrochemical cell is referred to as either an anode or a cathode
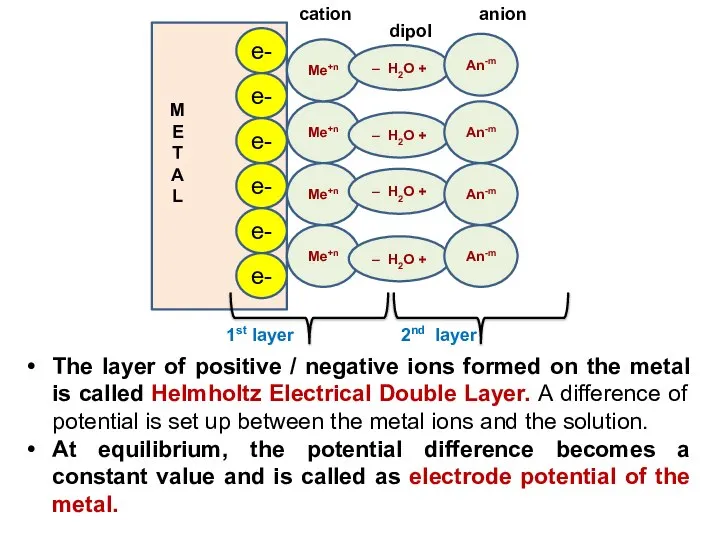
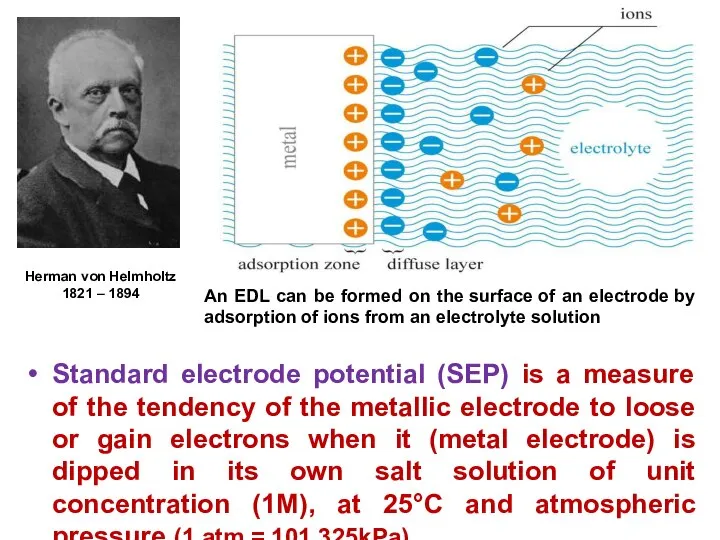
- 11. The layer of positive / negative ions formed on the metal is called Helmholtz Electrical Double
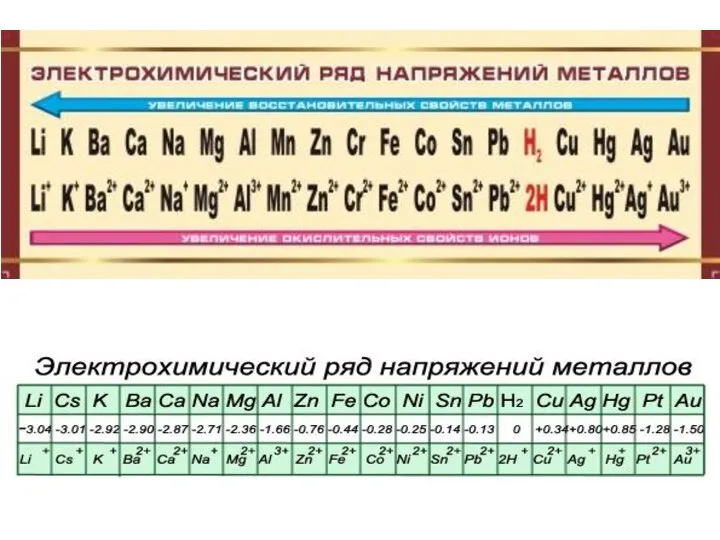
- 12. Standard electrode potential (SEP) is a measure of the tendency of the metallic electrode to loose
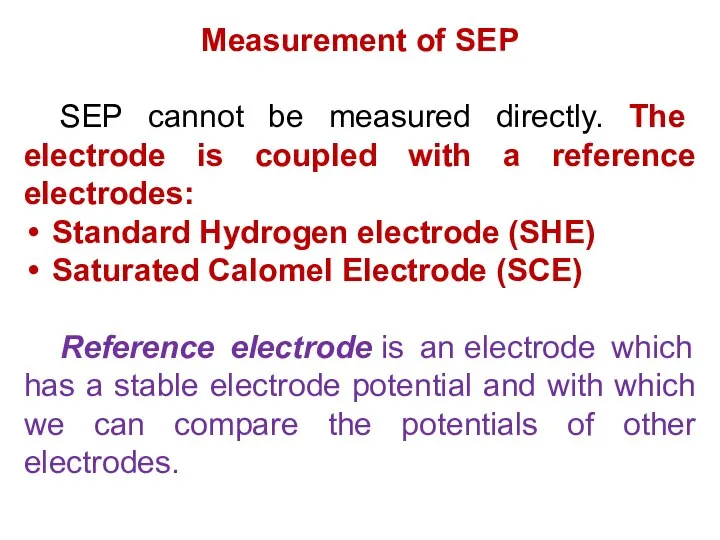
- 13. Measurement of SEP SEP cannot be measured directly. The electrode is coupled with a reference electrodes:
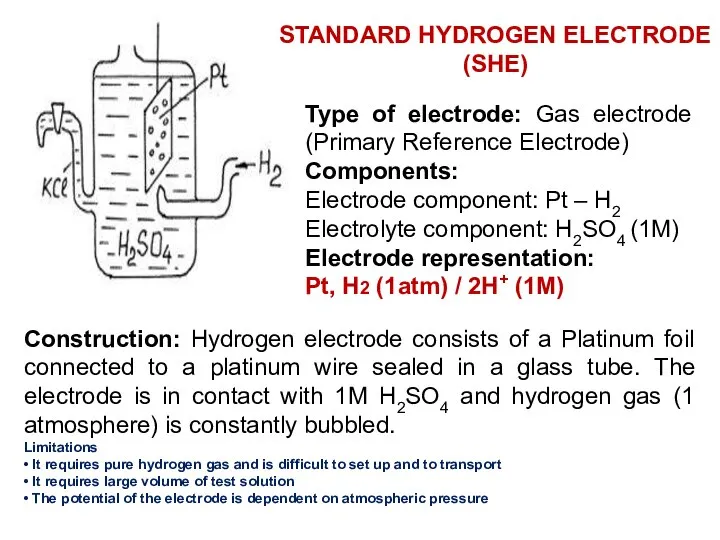
- 14. Type of electrode: Gas electrode (Primary Reference Electrode) Components: Electrode component: Pt – H2 Electrolyte component:
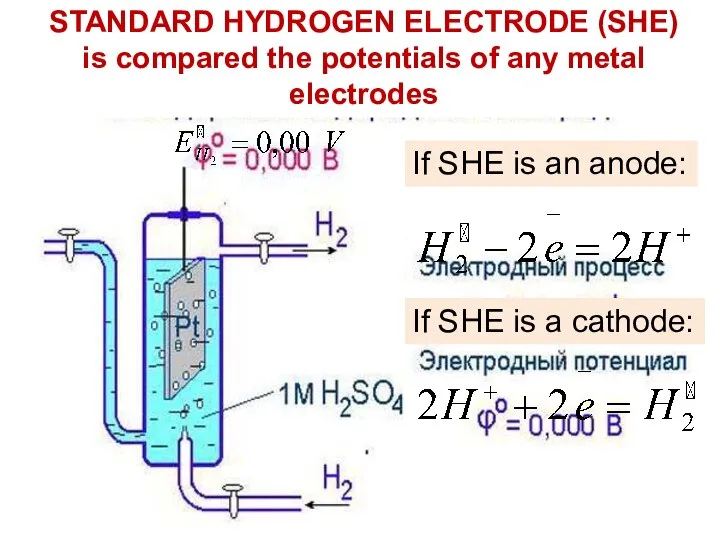
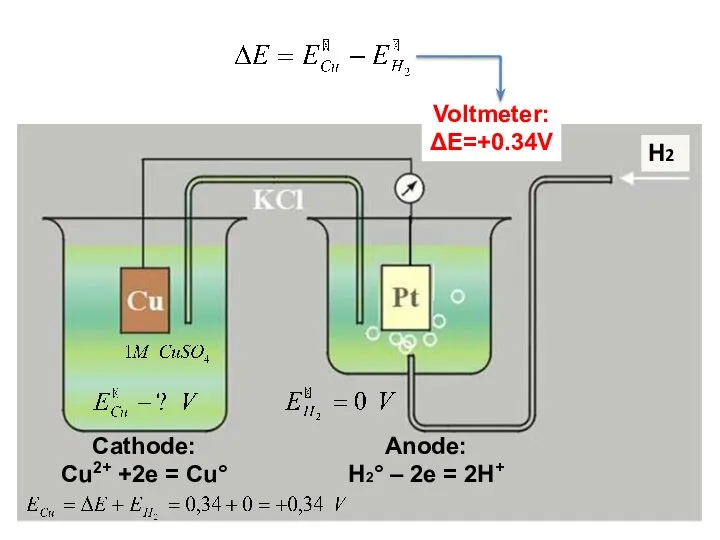
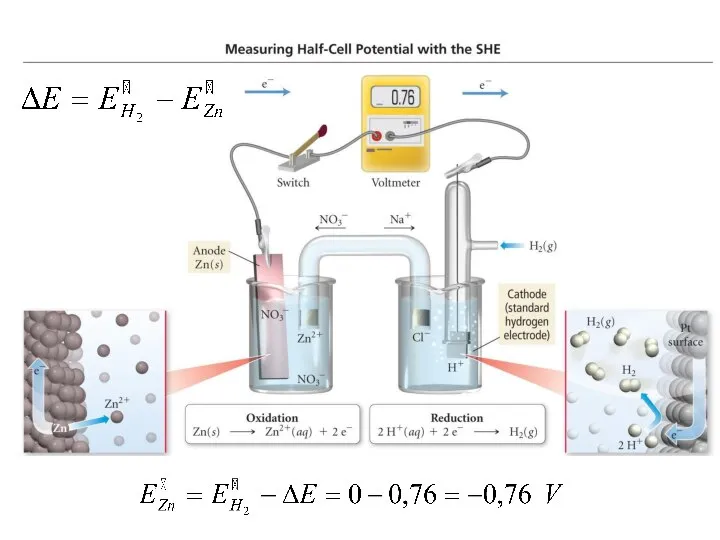
- 15. STANDARD HYDROGEN ELECTRODE (SHE) is compared the potentials of any metal electrodes If SHE is an
- 16. Cathode: Cu2+ +2e = Cu° Anode: H2° – 2e = 2H+ Н2 Voltmeter: ΔE=+0.34V
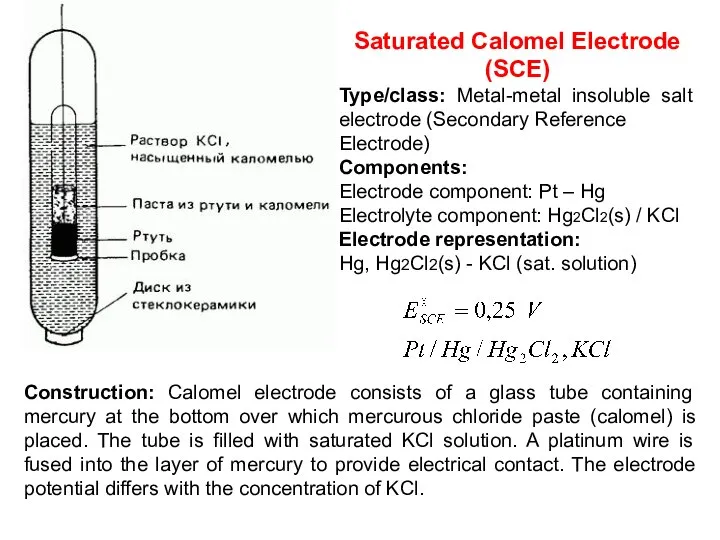
- 18. Saturated Calomel Electrode (SCE) Type/class: Metal-metal insoluble salt electrode (Secondary Reference Electrode) Components: Electrode component: Pt
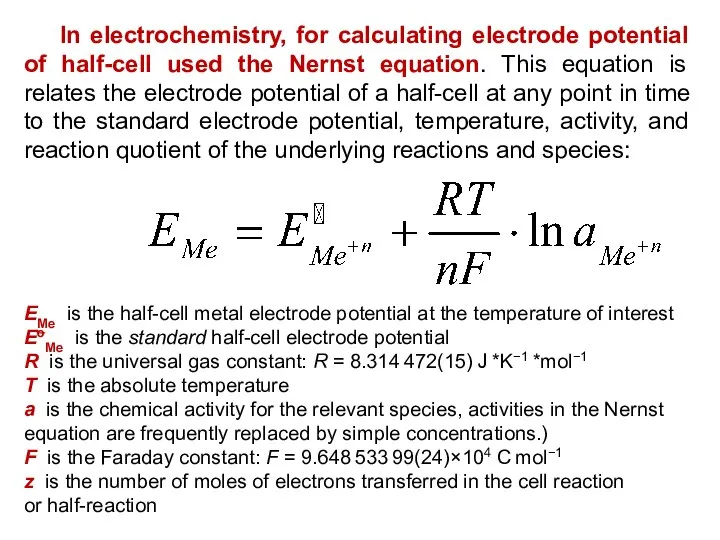
- 20. In electrochemistry, for calculating electrode potential of half-cell used the Nernst equation. This equation is relates
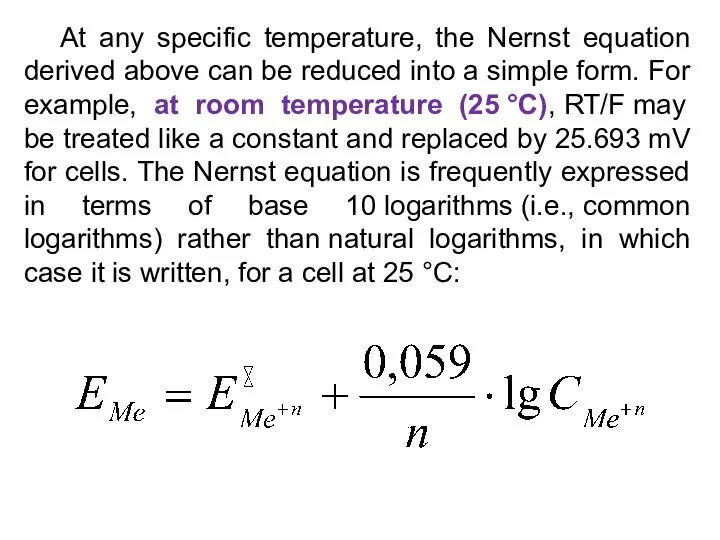
- 21. At any specific temperature, the Nernst equation derived above can be reduced into a simple form.
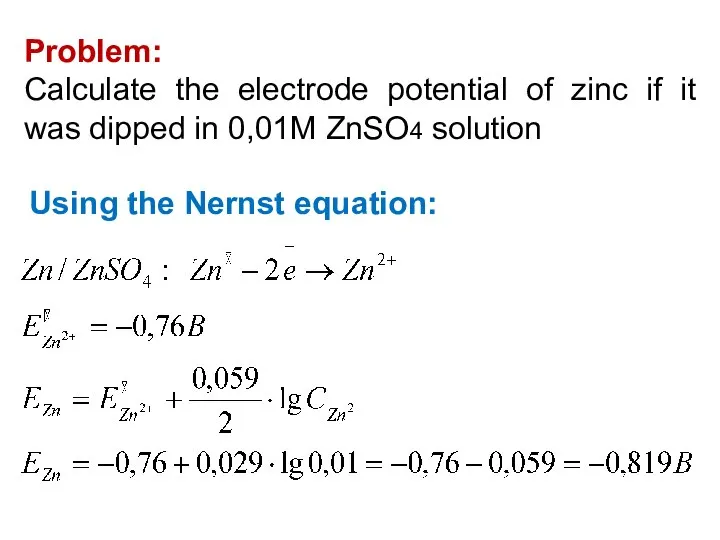
- 22. Problem: Calculate the electrode potential of zinc if it was dipped in 0,01M ZnSO4 solution Using
- 23. We know that reduction (gaining electrons) can’t happen without an oxidation to provide the electrons. When
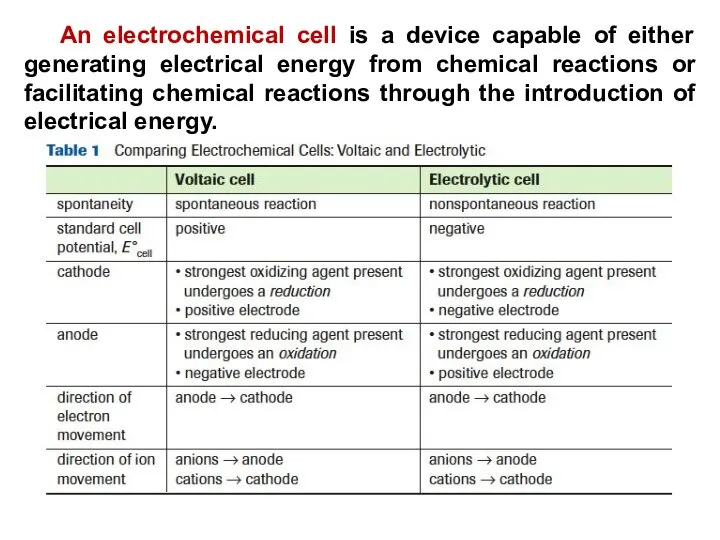
- 24. An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or
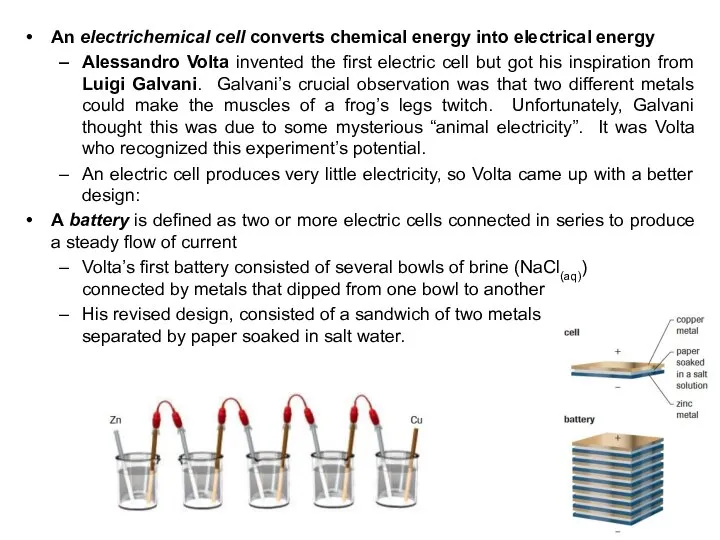
- 25. An electrichemical cell converts chemical energy into electrical energy Alessandro Volta invented the first electric cell
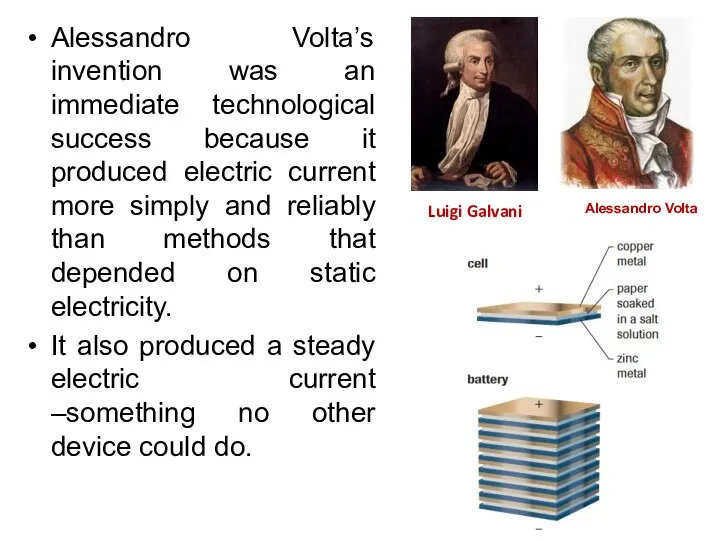
- 26. Alessandro Volta’s invention was an immediate technological success because it produced electric current more simply and
- 27. A galvanic cell, or voltaic cell, named after Luigi Galvani, or Alessandro Volta respectively, is an
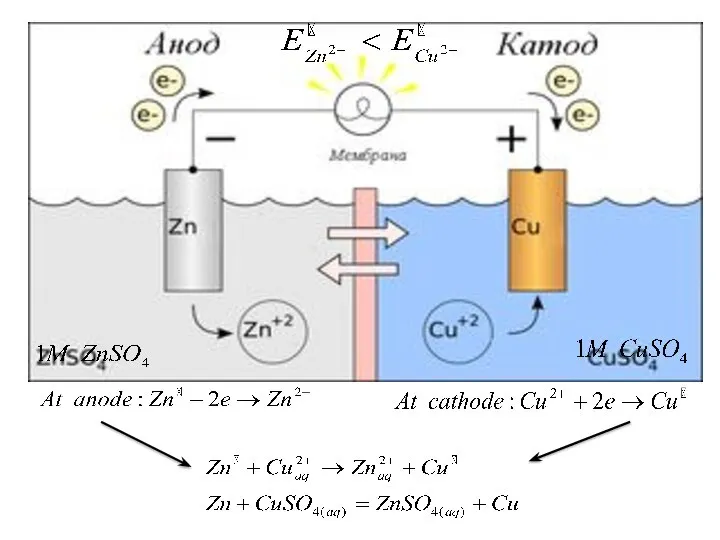
- 28. Galvanic cell composed of two half-cells; which each consist of a metal rod or strip immersed
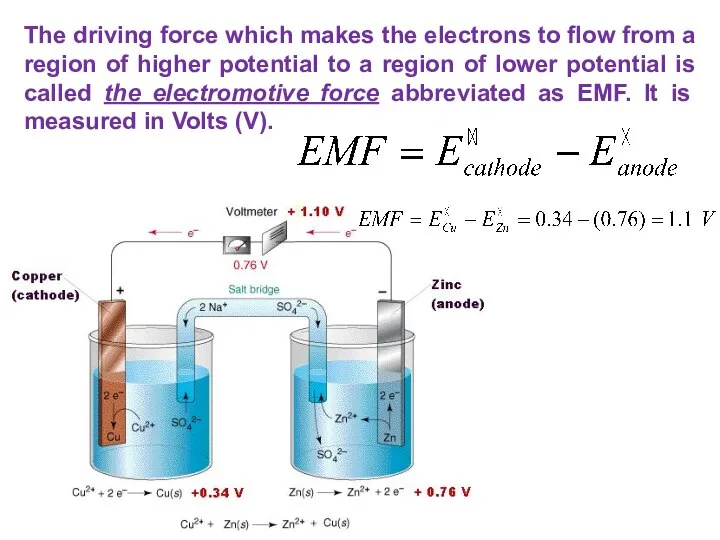
- 29. The driving force which makes the electrons to flow from a region of higher potential to
- 30. If the ΔE>0, it is positive, the reaction occurring is spontaneous. If the ΔE THE ELECTROMOTIVE
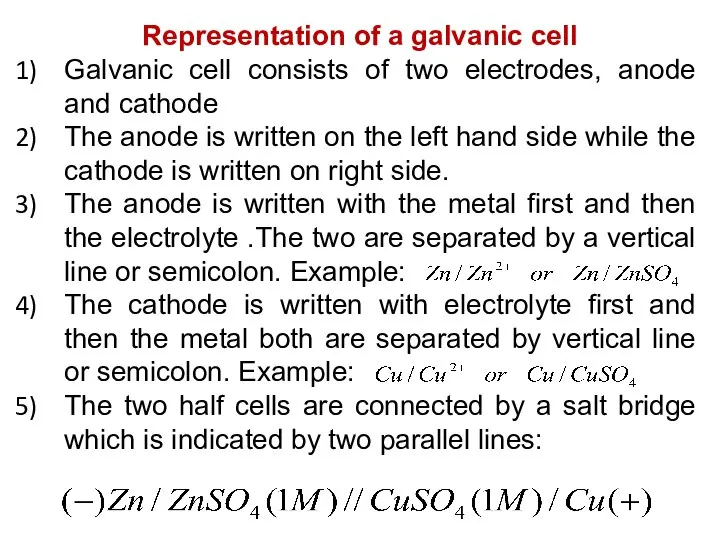
- 32. Representation of a galvanic cell Galvanic cell consists of two electrodes, anode and cathode The anode
- 33. Many natural phenomena are based on electrochemical processes, such as the corrosion of metals, the ability
- 35. Скачать презентацию







 Коллоидные растворы. Методы получения и очистки коллоидных растворов. Строение мицеллы гидрофобных систем. (Часть 1)
Коллоидные растворы. Методы получения и очистки коллоидных растворов. Строение мицеллы гидрофобных систем. (Часть 1) Соединения металлов. Оксиды и гидроксиды
Соединения металлов. Оксиды и гидроксиды Пурины. Строение пурина. (Лекция 9)
Пурины. Строение пурина. (Лекция 9) Промышленные аварии с выбросом опасных химических веществ
Промышленные аварии с выбросом опасных химических веществ Нефть и способы её переработки
Нефть и способы её переработки Физико-химия поверхностных явлений
Физико-химия поверхностных явлений Посвящение в химики
Посвящение в химики Безпечна для довкілля хімія «green chemistry». Основні напрямки та перспективи розвитку
Безпечна для довкілля хімія «green chemistry». Основні напрямки та перспективи розвитку Фізіологія мікроорганізмів. Живлення, ріст, розмноження, дихання, метаболізм. Вплив факторів зовнішнього середовища
Фізіологія мікроорганізмів. Живлення, ріст, розмноження, дихання, метаболізм. Вплив факторів зовнішнього середовища Сложные эфиры
Сложные эфиры Альдегиды и кетоны: свойства, получение, применение
Альдегиды и кетоны: свойства, получение, применение Открытый урок на тему: «Степень окисления»
Открытый урок на тему: «Степень окисления» Ароматические углеводороды. (Лекция 7)
Ароматические углеводороды. (Лекция 7) Одноатомные спирты. Химические свойства
Одноатомные спирты. Химические свойства Щелочные металлы
Щелочные металлы Геохимия редкоземельных элементов
Геохимия редкоземельных элементов Теоретические основы неорганического синтеза
Теоретические основы неорганического синтеза Презентация Углекислый газ СО2
Презентация Углекислый газ СО2  Характеристика хімічного елемента Hg
Характеристика хімічного елемента Hg Уроки зельеварения. Задача 6
Уроки зельеварения. Задача 6 солі в природі Солі препарати замінення атомів водної кислоти
солі в природі Солі препарати замінення атомів водної кислоти  Углеводы
Углеводы «Як би ми не зітхали до зірок, але електричної лампочки не покидаємось». І Вільде
«Як би ми не зітхали до зірок, але електричної лампочки не покидаємось». І Вільде  Презентация по Химии "Кальций" - скачать смотреть
Презентация по Химии "Кальций" - скачать смотреть  Ароматические амины
Ароматические амины Обмен триацилглицеролов и жирных кислот
Обмен триацилглицеролов и жирных кислот Структура основных тканей зуба. (Лекция 2)
Структура основных тканей зуба. (Лекция 2) Ph воды
Ph воды