How does a fuel cell work?
Hydrogen is a non-polluting fuel.
When it
burns in oxygen, water is the only product formed.
We can use this reaction to supply electrical energy continuously.
We do this by reaction hydrogen and oxygen in a fuel cell.
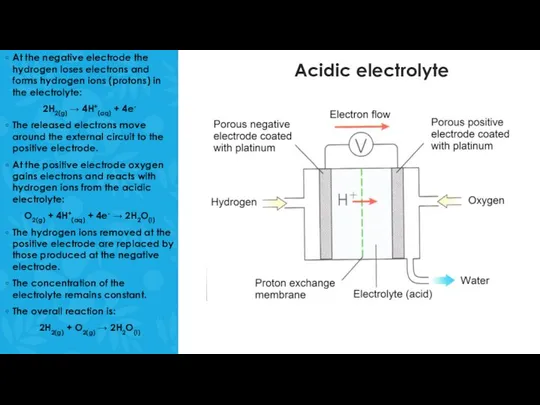
A fuel cell consists of two platinum electrodes and an electrolyte.
The platinum (Pt) is coated onto a porous material that allows gases to pass through it.
Hydrogen gas and oxygen gas are bubbled through the porous electrodes where the reaction take place.
Hydrogen gas is bubbled through the negative electrode and oxygen is bubbled through the positive electrode.
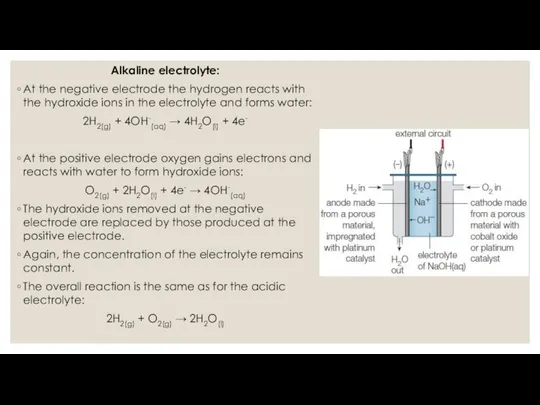
There are two main types of fuel cell.
One contains an acidic electrolyte, the other contains an alkaline electrolyte such as a concentrated solution of sodium hydroxide.









 Новые наноматериалы. Получение, свойства и применение
Новые наноматериалы. Получение, свойства и применение Дмитрий Иванович Менделеев
Дмитрий Иванович Менделеев Металдардың физикалық қасиеттерін ажырата білу
Металдардың физикалық қасиеттерін ажырата білу Химическое загрязнение акваторий. Основные термины и определения
Химическое загрязнение акваторий. Основные термины и определения Сера. Историческая справка
Сера. Историческая справка Поверхностные явления. Адсорбция
Поверхностные явления. Адсорбция Высокоэффективная жидкостная препаративная хроиматография
Высокоэффективная жидкостная препаративная хроиматография G11. Химический состав
G11. Химический состав Презентация к уроку окружающего мира по теме: ,,Про воздух и про воду” Цель урока: знакомство учеников с богатствами природы - воздухом и водой, их свойствами. Задачи: Продолжить расширение и углубление представлений
Презентация к уроку окружающего мира по теме: ,,Про воздух и про воду” Цель урока: знакомство учеников с богатствами природы - воздухом и водой, их свойствами. Задачи: Продолжить расширение и углубление представлений  Исследовательская работа по теме: « Волшебные жидкости – вещества определители или как определить вкус веществ не пробуя их»
Исследовательская работа по теме: « Волшебные жидкости – вещества определители или как определить вкус веществ не пробуя их»  Гетероциклді қосылыстар. Алкалоидтар
Гетероциклді қосылыстар. Алкалоидтар Электрохимиялық және химиялық коррозия
Электрохимиялық және химиялық коррозия Производные пиримидина
Производные пиримидина Плазменное состояние вещества
Плазменное состояние вещества Переработка нефти. (10 класс)
Переработка нефти. (10 класс) Получение дезинфицирующих средств
Получение дезинфицирующих средств Аттестационная работа. Методическая разработка спецкурса по химии для 8 класса Мир открытий. Вещества, окружающие нас в быту
Аттестационная работа. Методическая разработка спецкурса по химии для 8 класса Мир открытий. Вещества, окружающие нас в быту Галогены - друзья или враги?
Галогены - друзья или враги? Презентация по Химии "Вода в організмах" - скачать смотреть бесплатно
Презентация по Химии "Вода в організмах" - скачать смотреть бесплатно Składniki odżywcze
Składniki odżywcze Алмазы, искусственный и естественный рост
Алмазы, искусственный и естественный рост Презентация по Химии "Химические антонимы" - скачать смотреть
Презентация по Химии "Химические антонимы" - скачать смотреть  Общая характеристика неметаллов
Общая характеристика неметаллов Адсорбционные равновесия и процессы на подвижных и неподвижных границах раздела фаз
Адсорбционные равновесия и процессы на подвижных и неподвижных границах раздела фаз Электролиты и неэлектролиты
Электролиты и неэлектролиты Химия вопросы и ответы
Химия вопросы и ответы Биосинтез заменимых аминокислот. Деградация нуклеиновых кислот. (Лекция 4)
Биосинтез заменимых аминокислот. Деградация нуклеиновых кислот. (Лекция 4) Сера и ее соединения
Сера и ее соединения