Содержание
- 2. Antoine Henri Becquerel (1852-1908)
- 3. Henri Becquerel was born into a family of scientists. His grandfather had made important contributions in
- 4. And so, upon learning how Wilhelm Roentgen discovered x-rays from the fluorescence they produced, Becquerel had
- 5. The material Becquerel chose to work with was potassium uranyl sulphate, K2UO2 (SO4 )2, which he
- 6. Becquerel concluded “that the phosphorescence substance in question emits radiation which penetrates paper opaque to light”.
- 8. Further investigation, on the 26th and 27th of February, was delayed because the skies over Paris
- 9. On the first of March, he developed the photographic plates expecting only faint images to appear.
- 10. This meant that the uranium emitted radiation without an external source of energy such as the
- 11. Later, Becquerel demonstrated that the radiation emitted by uranium shared certain characteristic with x-rays but, unlike
- 12. Pierre Curie (1859-1906) Marie Curie (1867-1934)
- 13. Pierre Curie and Marie Curie began investigating the phenomenon of radioactivity recently discovered in uranium ore.
- 14. She concluded that the ore contained, in addition to uranium, new elements that were also radioactive.
- 15. For their work on radioactivity, the Curies were awarded the 1903 Nobel Prize in physics. Tragically,
- 16. Pierre’s teaching position at the Sorbonne was given to Marie. Never before had a woman taught
- 17. Ernest Rutherford (1871-1937)
- 18. Ernest Rutherford is considered the father of nuclear physics. Indeed, it could be said that Rutherford
- 19. Even the neutron, discovered by James Chadwick, owes its name to Rutherford. Purpose by Rutherford and
- 20. For this work, Rutherford won the 1908 Nobel Prize in chemistry. In 1909, now at the
- 21. From this simple observation, Rutherford concluded that the atom’s mass must be concentrated in a small
- 22. In 1919, Rutherford returned to Cambridge to become director of the Cavendish laboratory where he had
- 23. It was here that he made his final major achievement, the artificial alteration of nuclear and
- 24. What is ionizing radiation? Ionizing radiation is radiation that has sufficient energy to remove orbital electrons
- 25. One source of radiation is the nuclei of unstable atoms. For these radioactive atoms (also referred
- 26. Unstable isotopes of radium, radon, uranium, and thorium, for example, exist naturally. Others are continually being
- 27. Types of ionizing radiation alpha particle radiation beta particle radiation gamma ray radiation x-ray Radiation
- 28. Alpha Particle Radiation An alpha particle consists of two neutrons and two protons ejected from the
- 29. The a-rays are positively charged. Because alpha particles are charged and relatively heavy, they interact intensely
- 30. Alpha particles are easily shielded against and can be stopped by a single sheet of paper.
- 31. However, due to the very large number of ionizations they produce in a very short distance,
- 32. Beta Particle Radiation A beta particle is an electron emitted from the nucleus of a radioactive
- 33. Beta particles are much less massive and less charged than alpha particles and interact less intensely
- 34. Some energetic beta particles, such as those from P-32 (phosphorus), will travel up to several feet
- 35. All beta emitters, depending on the amount present, can pose a hazard if inhaled, ingested or
- 36. Gamma Ray Radiation A gamma ray is a packet (or photon) of electromagnetic radiation emitted from
- 37. Gamma rays are identical in nature to other electromagnetic radiations such as light or microwaves but
- 38. Like all forms of electromagnetic radiation, gamma rays have no mass or charge and interact less
- 39. Gamma radiation is typically shielded using very dense materials (the denser the material, the more chance
- 40. X-Ray Radiation Like a gamma ray, an x-ray is a packet (or photon) of electromagnetic radiation
- 41. X-rays are produced as the result of changes in the positions of the electrons orbiting the
- 42. X-rays can be produced during the process of radioactive decay or as bremsstrahlung radiation. Bremsstrahlung radiation
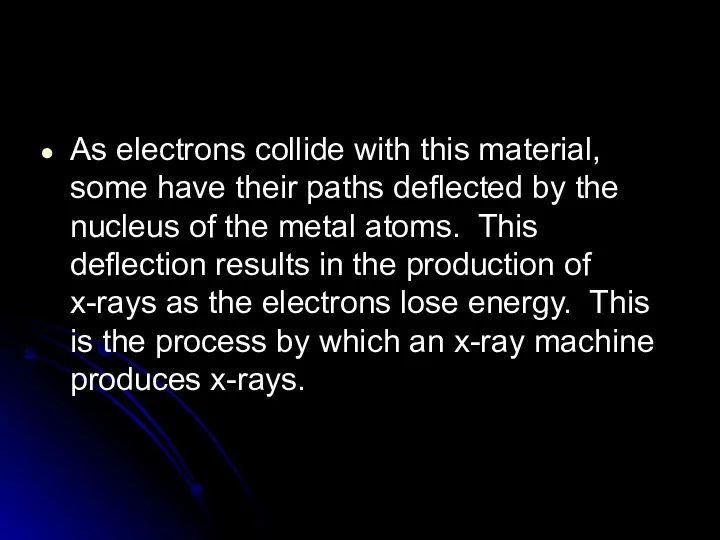
- 43. As electrons collide with this material, some have their paths deflected by the nucleus of the
- 44. Like gamma rays, x-rays are typically shielded using very dense materials such as lead or other
- 45. Non-ionizing Radiation Nonionizing radiations are not energetic enough to ionize atoms and interact with materials in
- 46. Examples of nonionizing radiation include: Microwaves Visible Light Radio Waves TV Waves Ultraviolet Light
- 47. Natural radioactivity Definition: it is defined as the radioactivity displayed by natural isotopes of elements. For
- 48. Artificial radioactivity Definition: artificial radioactivity is defined as the process of changing common stable nuclei of
- 49. Fredric and Irene Curie shared the 1935 Nobel Prize in chemistry for their investigations on the
- 50. Natural background radiation To put these radiation effects into perspective, it is worth looking at the
- 51. By natural radiations, we mean those radiations within the environment over which we have no control
- 52. For example, cosmic radiations bombard the earth from outer space and their intensity will depend on
- 53. Our exposure to cosmic radiation will therefore depend on the altitude at which we live and
- 54. The major source of “natural” radiation is the gas radon. Radon permeates through the rocks into
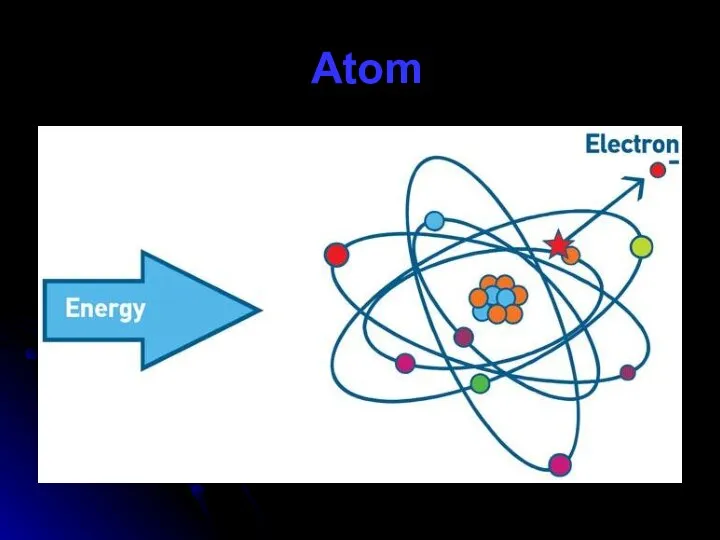
- 55. Atom
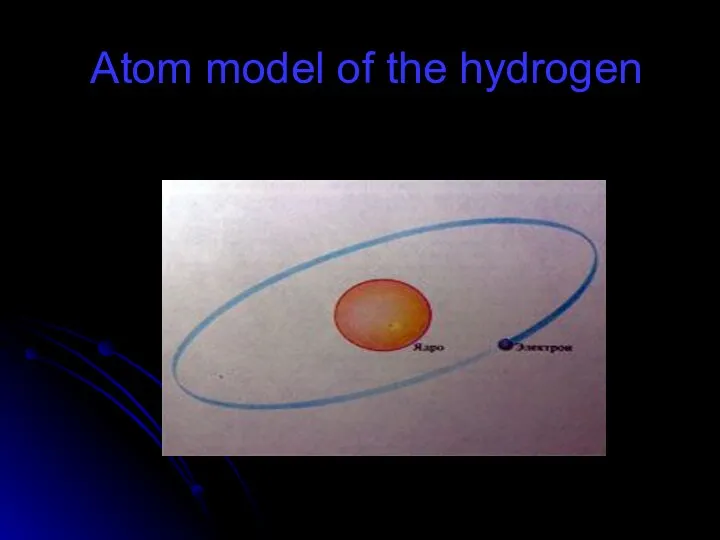
- 56. Atom model of the hydrogen
- 57. Atom model There are three parts of an atom: protons neutron electrons
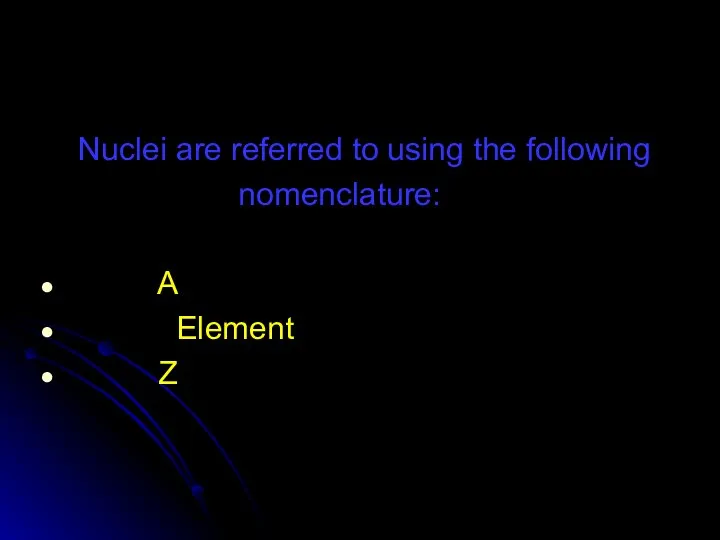
- 58. Nuclei are referred to using the following nomenclature: A Element Z
- 59. Z is the atomic number. It characterizes the element. It also the number of protons. Since
- 60. A is called the “mass number” and is equal to the sum of Z and neutrons.
- 61. A species of nucleus of given Z and A is called nuclide. Nuclides of an element
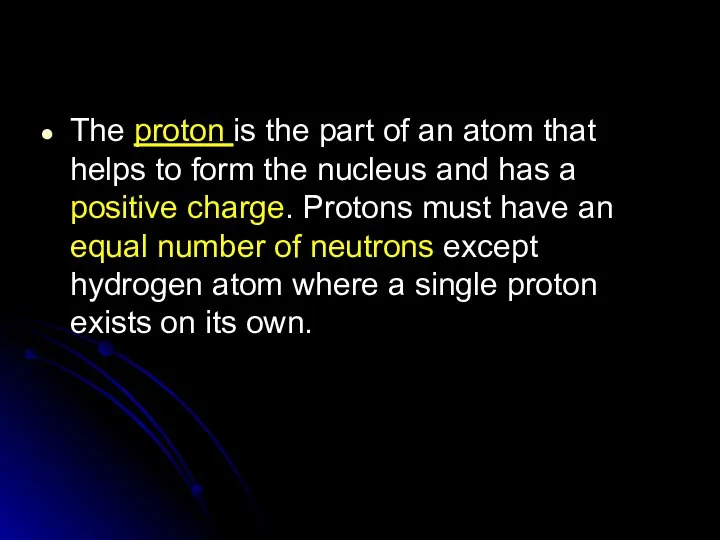
- 62. The proton is the part of an atom that helps to form the nucleus and has
- 63. A neutron is the part of an atom that holds no charge. Neutrons and protons occur
- 64. Electrons are the smallest parts of the atom and have a negative charge. They are the
- 65. The positron is identical to the electron except that it carries opposite charge. When an electron
- 66. Radioactive decay
- 67. Radioactive decay is the process in which an unstable atomic nucleus spontaneously loses energy by emitting
- 68. For example: a carbon-14 atom (the “parent”) emits radiation and transforms to a nitrogen-14 atom (the
- 69. The SI (international system) unit of activity is the bequerel (Bq). One Bq is defined as
- 70. Half-life Half-life is the period of time it takes for a substance undergoing decay to decrease
- 71. For example: consider 10 kg of radioelement with a half-life of 1 hour. In the first
- 72. Initially, the rate of disintegration is rapid, but it becomes slower as time passes. The fraction
- 73. Therefore, for comparison between different radioactive substances we consider the quantity called the half-life of the
- 74. Radiation protection principle There are four basic radiation protection principles that can be employed to reduce
- 75. Time Time is an important factor in radiation protection. The principles states that the shorter the
- 76. Many radiation monitoring devices measure exposure in milliroentgens (mR) per hour. An exposure rate of 60
- 77. Distance The second radiation protection factor is distance, and the principle is the farther a person
- 78. For example, a source of radiation that measures 8 mR/hr at 2 feet a source would
- 79. Shielding The third radiation protection factor is shielding. The principle follows that the denser a material,
- 80. In addition, some specialty centers for radiation accident management have constructed shield surgical tables for protection.
- 81. In emergency management of the contaminated patient, shielding is limited to standard surgical clothing with slight
- 82. However, it does not stop penetrating gamma radiation. In the hospital emergency department shielding is actually
- 83. Quantity The fourth radiation protection factor is quantity. Because the exposure rate from a given radioactive
- 84. At work with the closed sources of radiations there is a potential danger of radioactive pollution
- 85. Check of tightness of the closed sources is necessary for carrying out on a regular basis
- 87. Скачать презентацию














































































 6-я группа элементов. 9 класс
6-я группа элементов. 9 класс Алкадиены. Классификация алкадиенов
Алкадиены. Классификация алкадиенов Нуклеиновые кислоты
Нуклеиновые кислоты Презентация по Химии "Степень окисления" - скачать смотреть бесплатно_
Презентация по Химии "Степень окисления" - скачать смотреть бесплатно_ Сырьё для получения фенолальдегидных полимеров
Сырьё для получения фенолальдегидных полимеров Презентация по Химии "Увлекательные факты из жизни великого химика." - скачать смотреть бесплатно
Презентация по Химии "Увлекательные факты из жизни великого химика." - скачать смотреть бесплатно Разработка и исследование системы управления процессом осушки бутанола при производстве н -бутилового спирта
Разработка и исследование системы управления процессом осушки бутанола при производстве н -бутилового спирта Химическая кинетика и катализ
Химическая кинетика и катализ Ферросплавы. Феррохром
Ферросплавы. Феррохром Закон Авогадро. Молярный объем и относительные плотности газов. Уравнение Клапейрона-Менделеева
Закон Авогадро. Молярный объем и относительные плотности газов. Уравнение Клапейрона-Менделеева Нефть. Свойства, состав, переработка
Нефть. Свойства, состав, переработка Синтез ультрадисперсного мела для различных эффективных использований
Синтез ультрадисперсного мела для различных эффективных использований К 180-летию со дня рождения Д.И. Менделеева
К 180-летию со дня рождения Д.И. Менделеева Кислоты, их классификация и свойства Презентация к уроку химии для 8 класса Учитель МОУ «Куженерская средняя общеобразовате
Кислоты, их классификация и свойства Презентация к уроку химии для 8 класса Учитель МОУ «Куженерская средняя общеобразовате Электроды 1 рода
Электроды 1 рода Homecredit Bank. Показатели и зоны роста
Homecredit Bank. Показатели и зоны роста Закон действующих масс и его применение к различным типам равновесий
Закон действующих масс и его применение к различным типам равновесий ПОЛІПРОПІЛЕН ( РР) СИНТЕТИЧНИЙ ПОЛІМЕР, ПРОДУКТ ПОЛІМЕРИЗАЦІЇ ПРОПІЛЕНУ, [—СН2—СН(СН3)—]N.
ПОЛІПРОПІЛЕН ( РР) СИНТЕТИЧНИЙ ПОЛІМЕР, ПРОДУКТ ПОЛІМЕРИЗАЦІЇ ПРОПІЛЕНУ, [—СН2—СН(СН3)—]N.  Газоанализаторы. Измерительный прибор для определения качественного и количественного состава смесей газов
Газоанализаторы. Измерительный прибор для определения качественного и количественного состава смесей газов Посуда, ее виды и использование. Работу выполнила Гливинская Анастасия ученица 9 класса МБОУ «СОШ №11»
Посуда, ее виды и использование. Работу выполнила Гливинская Анастасия ученица 9 класса МБОУ «СОШ №11»  Нитраты, их влияние на организм человека
Нитраты, их влияние на организм человека Поливинилхлорид. Физические и химические свойства
Поливинилхлорид. Физические и химические свойства Азот. Открытие азота
Азот. Открытие азота Предмет химии. Вещества. 8 клвсс
Предмет химии. Вещества. 8 клвсс Тіндер-тірі организмнің иерархиялық деңгейінің ұйымдастырудың бір түрі. Олардың құрылымдық принциптері
Тіндер-тірі организмнің иерархиялық деңгейінің ұйымдастырудың бір түрі. Олардың құрылымдық принциптері Химическая термодинамика
Химическая термодинамика AgCl негізіндегі нанокомпазиттердің фотокаталитикалық белсенділігі
AgCl негізіндегі нанокомпазиттердің фотокаталитикалық белсенділігі Сахароза
Сахароза