Содержание
- 2. Prentice-Hall © 2002 General Chemistry: Chapter 1 Slide of 19 Contents Physical properties and states of
- 3. Prentice-Hall © 2002 General Chemistry: Chapter 1 Slide of 19 Properties of Matter Matter: Occupies space,
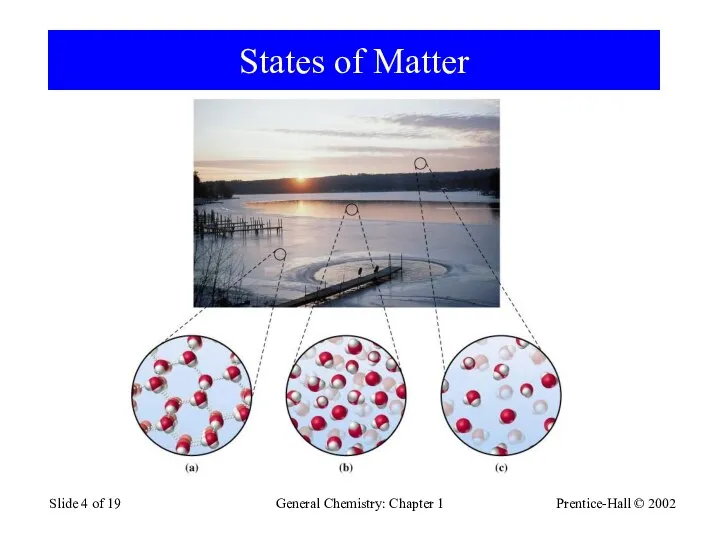
- 4. Prentice-Hall © 2002 General Chemistry: Chapter 1 Slide of 19 States of Matter
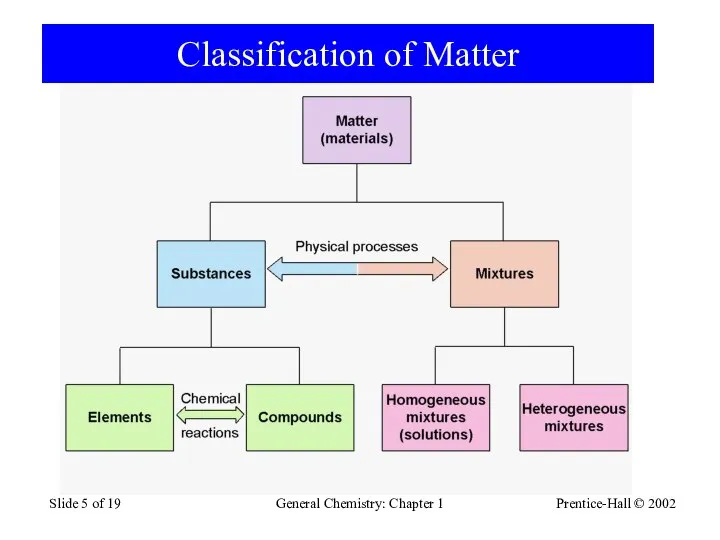
- 5. Prentice-Hall © 2002 General Chemistry: Chapter 1 Slide of 19 Classification of Matter
- 6. Prentice-Hall © 2002 General Chemistry: Chapter 1 Slide of 19 Separations
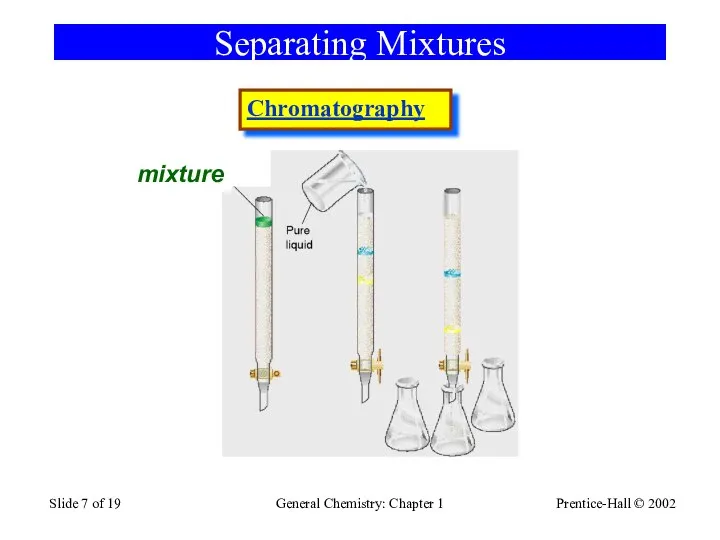
- 7. Prentice-Hall © 2002 General Chemistry: Chapter 1 Slide of 19 Separating Mixtures mixture Chromatography
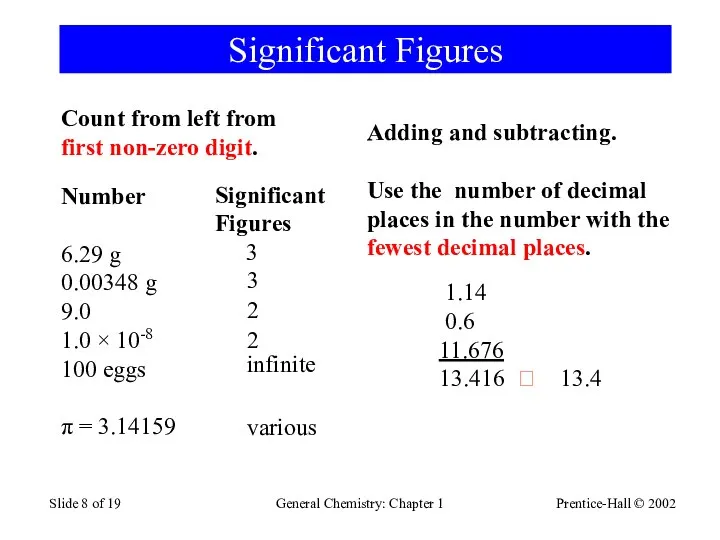
- 8. Prentice-Hall © 2002 General Chemistry: Chapter 1 Slide of 19 Significant Figures Number 6.29 g 0.00348
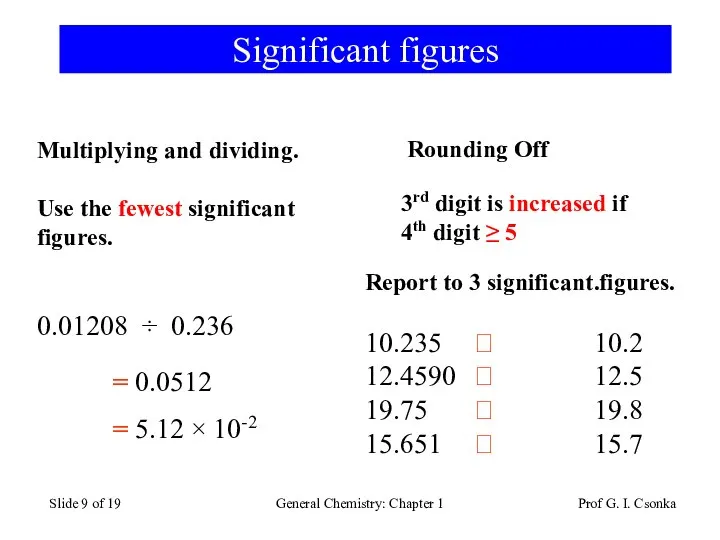
- 9. Prof G. I. Csonka General Chemistry: Chapter 1 Slide of 19 Significant figures Multiplying and dividing.
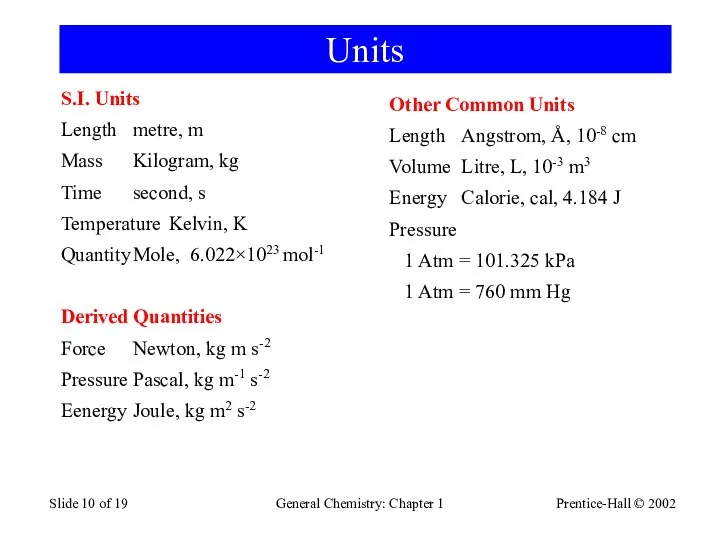
- 10. Prentice-Hall © 2002 General Chemistry: Chapter 1 Slide of 19 Units S.I. Units Length metre, m
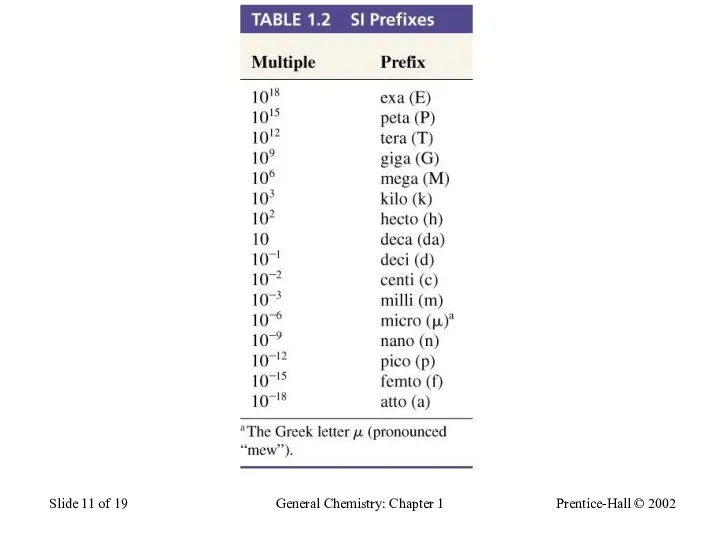
- 11. Prentice-Hall © 2002 General Chemistry: Chapter 1 Slide of 19
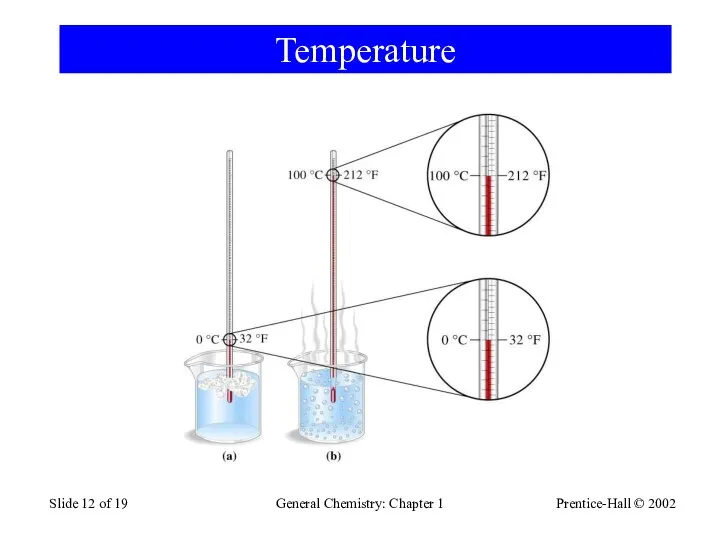
- 12. Prentice-Hall © 2002 General Chemistry: Chapter 1 Slide of 19 Temperature
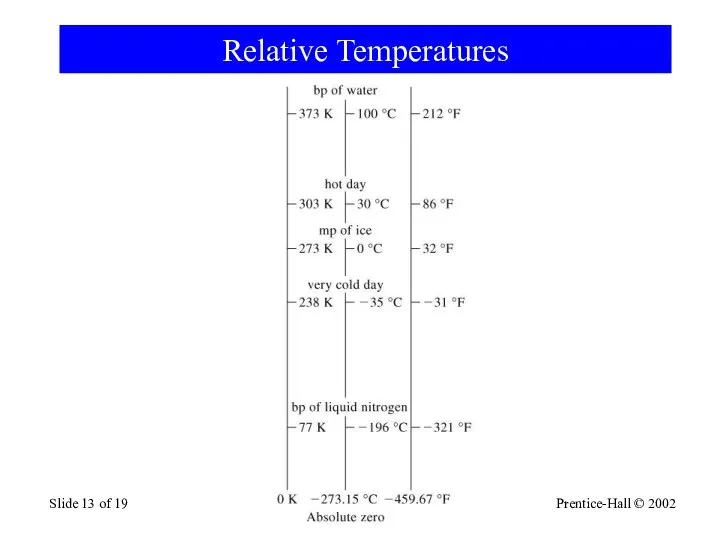
- 13. Prentice-Hall © 2002 General Chemistry: Chapter 1 Slide of 19 Relative Temperatures
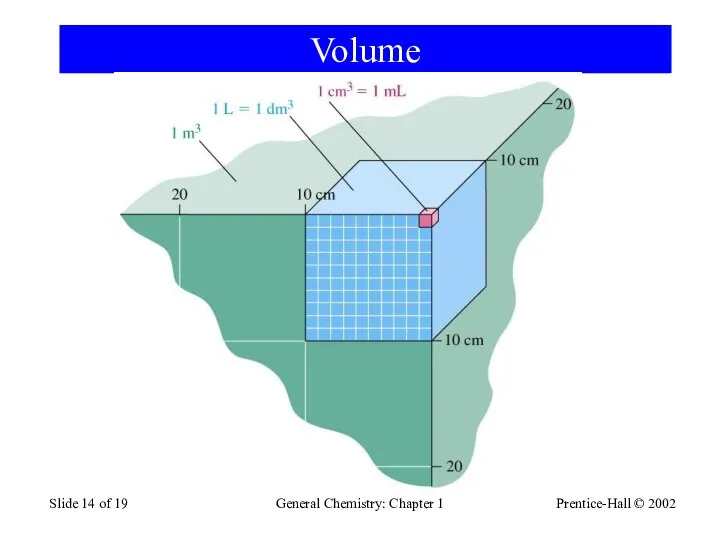
- 14. Prentice-Hall © 2002 General Chemistry: Chapter 1 Slide of 19 Volume
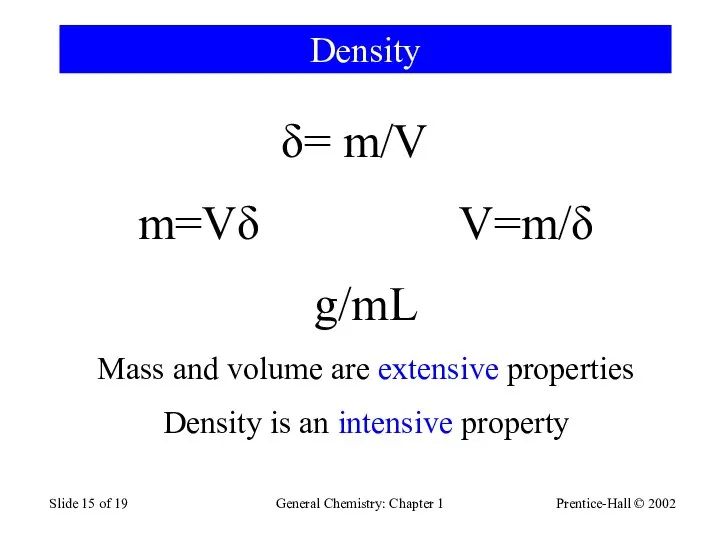
- 15. Prentice-Hall © 2002 General Chemistry: Chapter 1 Slide of 19 Density = m/V m=Vδ V=m/δ g/mL
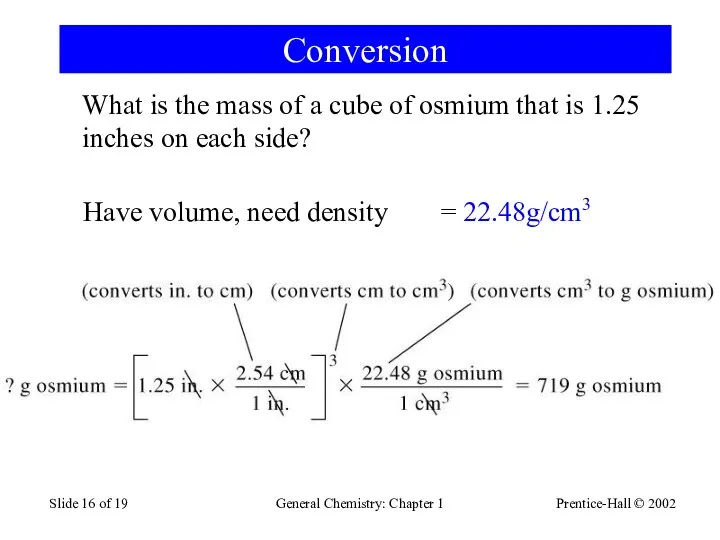
- 16. Prentice-Hall © 2002 General Chemistry: Chapter 1 Slide of 19 Conversion What is the mass of
- 17. Prentice-Hall © 2002 General Chemistry: Chapter 1 Slide of 19 Wrong units
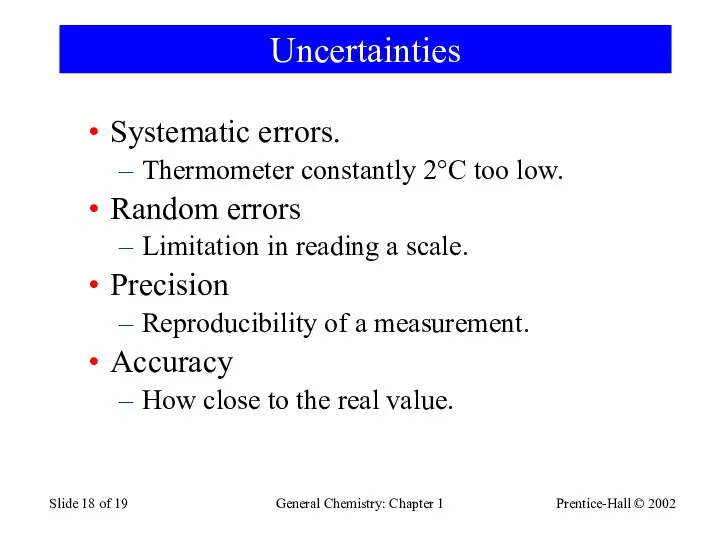
- 18. Prentice-Hall © 2002 General Chemistry: Chapter 1 Slide of 19 Uncertainties Systematic errors. Thermometer constantly 2°C
- 20. Скачать презентацию


 Презентация по Химии "Миючі засоби в побуті. Мило. Првальні порошки" - скачать смотреть бесплатно
Презентация по Химии "Миючі засоби в побуті. Мило. Првальні порошки" - скачать смотреть бесплатно Дегтярева М.О. Московская область г. Королёв АОУ ЛНИП 242-645-771
Дегтярева М.О. Московская область г. Королёв АОУ ЛНИП 242-645-771 Термопластичні Термореактивні
Термопластичні Термореактивні 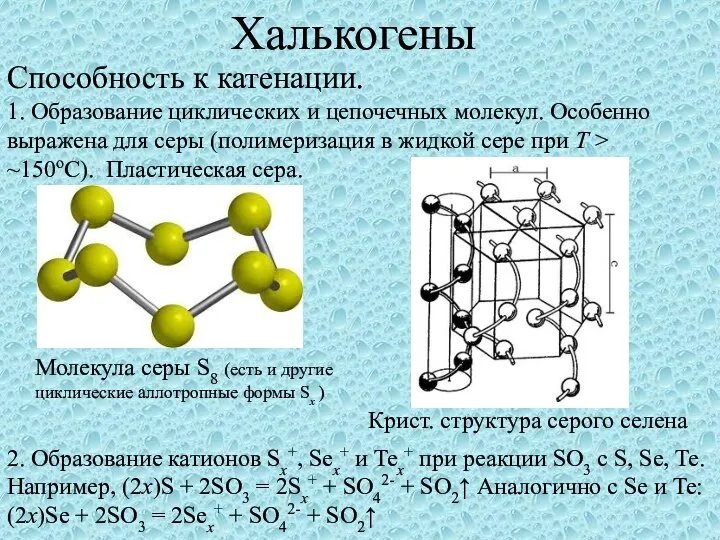 Халькогены. Способность к катенации
Халькогены. Способность к катенации Душистые вещества животного и растительного происхождения
Душистые вещества животного и растительного происхождения Донорно-акцепторный механизм образования ковалентной связи. Комплексные соединения. Лекция 9
Донорно-акцепторный механизм образования ковалентной связи. Комплексные соединения. Лекция 9 Презентация по Химии "Получение водорода в лаборатории" - скачать смотреть
Презентация по Химии "Получение водорода в лаборатории" - скачать смотреть  Металлы и сплавы
Металлы и сплавы Секреты химии. Проект Радуга
Секреты химии. Проект Радуга Дисперсные системы
Дисперсные системы Брейн-ринг. Відгадай елемент
Брейн-ринг. Відгадай елемент Закон сохранения массы веществ. Уравнения химических реакций
Закон сохранения массы веществ. Уравнения химических реакций Теория растворов (лекция 2)
Теория растворов (лекция 2) Исследование воздействия плазмы метана на свойства оксида графена
Исследование воздействия плазмы метана на свойства оксида графена Строение электронных оболочек атомов
Строение электронных оболочек атомов Выбраковка результатов химического анализа
Выбраковка результатов химического анализа Электронное строение атома
Электронное строение атома Презентация по Химии "Кислород" - скачать смотреть
Презентация по Химии "Кислород" - скачать смотреть  Кристаллические решетки
Кристаллические решетки Біоорганічна хімія. Реакційна здатність біоорганічних сполук
Біоорганічна хімія. Реакційна здатність біоорганічних сполук Алкени (етиленові вуглеводні, олефіни)
Алкени (етиленові вуглеводні, олефіни)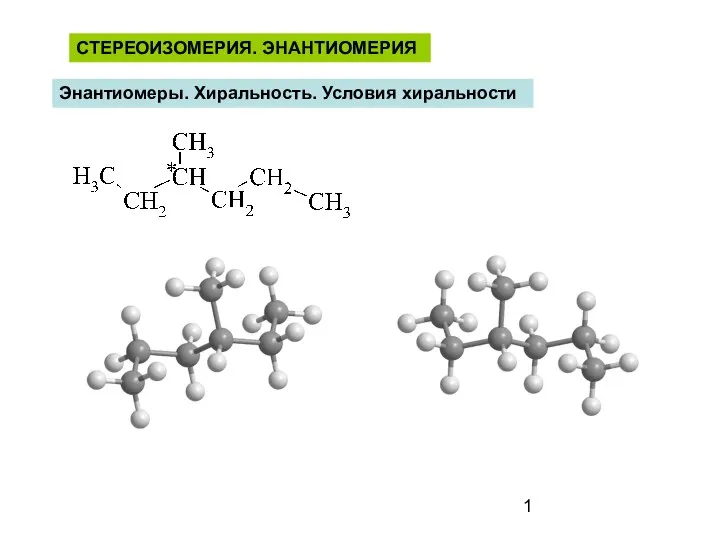 Стереоизомерия. Энантиомерия. Энантиомеры. Хиральность. Условия хиральности
Стереоизомерия. Энантиомерия. Энантиомеры. Хиральность. Условия хиральности Определение крахмала в продуктах
Определение крахмала в продуктах Синтез и химические модификации индиго
Синтез и химические модификации индиго Метаболизм - обмен веществ
Метаболизм - обмен веществ Кристаллизации металлов. Методы исследования металлов
Кристаллизации металлов. Методы исследования металлов Доронькин Владимир Николаевич, кандидат химических наук, доцент РГУПС, автор пособий по химии издательства «Легион»
Доронькин Владимир Николаевич, кандидат химических наук, доцент РГУПС, автор пособий по химии издательства «Легион»  Окислительно-восстановительные реакции
Окислительно-восстановительные реакции