Содержание
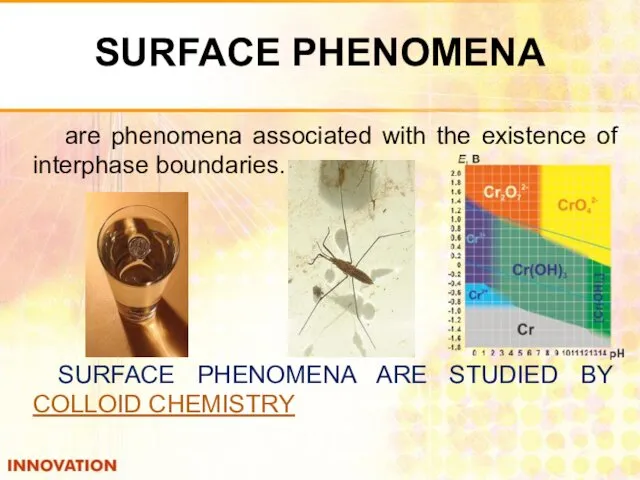
- 2. SURFACE PHENOMENA are phenomena associated with the existence of interphase boundaries. SURFACE PHENOMENA ARE STUDIED BY
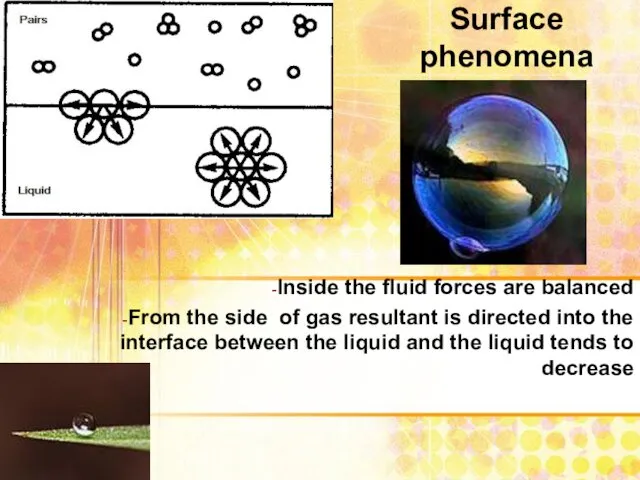
- 3. Surface phenomena Inside the fluid forces are balanced From the side of gas resultant is directed
- 4. Surface phenomena The increased surface area of the phase separation is associated with the transition of
- 5. Surface tension σ is the work required for the creation of 1 m2 of surface [σ]=
- 6. Surface tension Surface tension depends on: the nature of fluid σ(Н2О)=72,8 J/m2; σ(serum)=45,4 J/m2). temperature (↑t
- 7. SORPTION
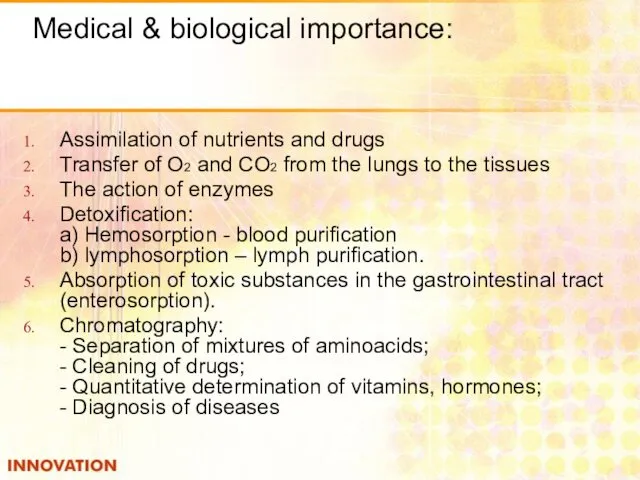
- 8. Medical & biological importance: Assimilation of nutrients and drugs Transfer of O2 and CO2 from the
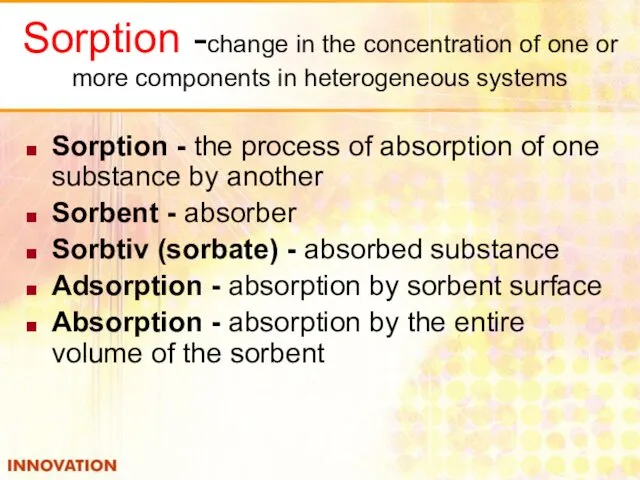
- 9. Sorption -change in the concentration of one or more components in heterogeneous systems Sorption - the
- 10. Adsorption Adsorption is spontaneous change of component concentration in the surface layer compared to the volume
- 11. Gibbs Equation G - the amount of adsorbed substance [mole/m2] а – equilibrium activity of the
- 12. Surface activity The ability of the solute to change surface tension is called surface activity (γ)
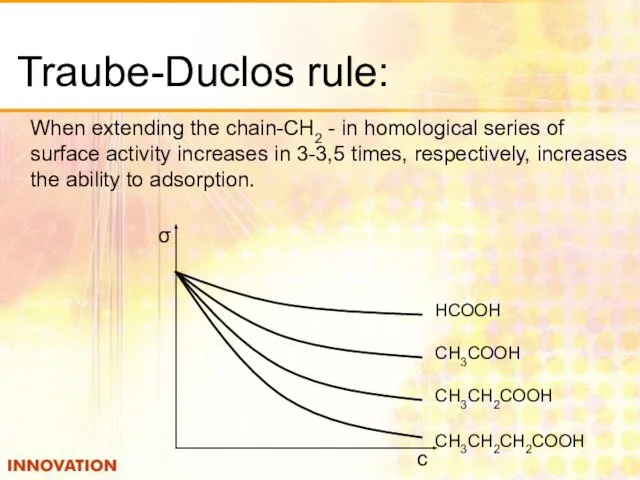
- 13. Traube-Duclos rule: When extending the chain-CH2 - in homological series of surface activity increases in 3-3,5
- 14. SAS, SIS, SNS Surface-active substances (SAS): reduce σ solvent. σ solution O. SAS: alcohols, organic acids,
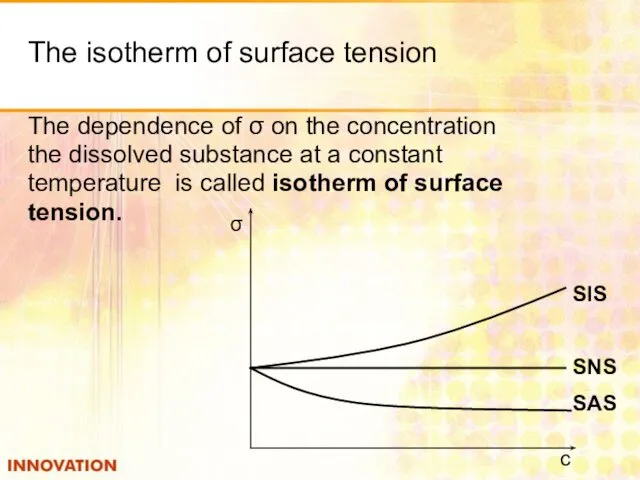
- 15. The isotherm of surface tension The dependence of σ on the concentration the dissolved substance at
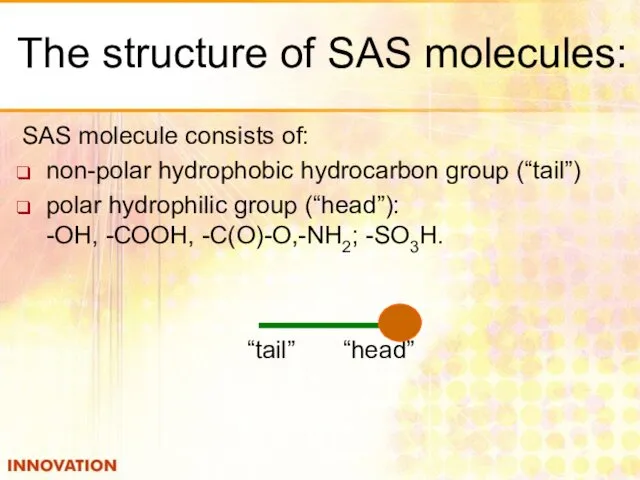
- 16. The structure of SAS molecules: SAS molecule consists of: non-polar hydrophobic hydrocarbon group (“tail”) polar hydrophilic
- 17. ADSORPTION ON THE SOLUTION-GAS BORDER
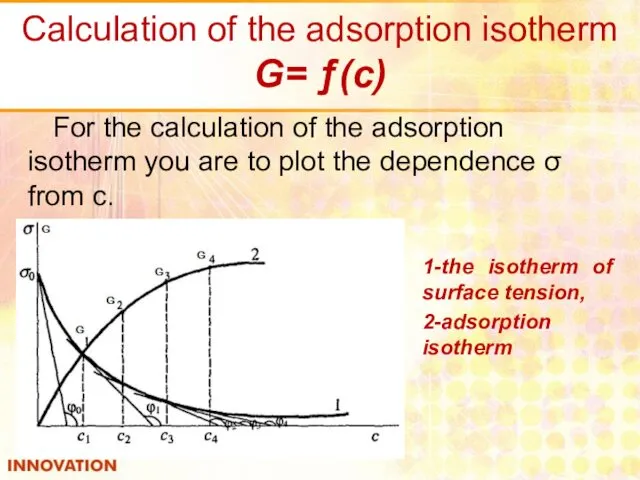
- 18. Calculation of the adsorption isotherm G= ƒ(с) For the calculation of the adsorption isotherm you are
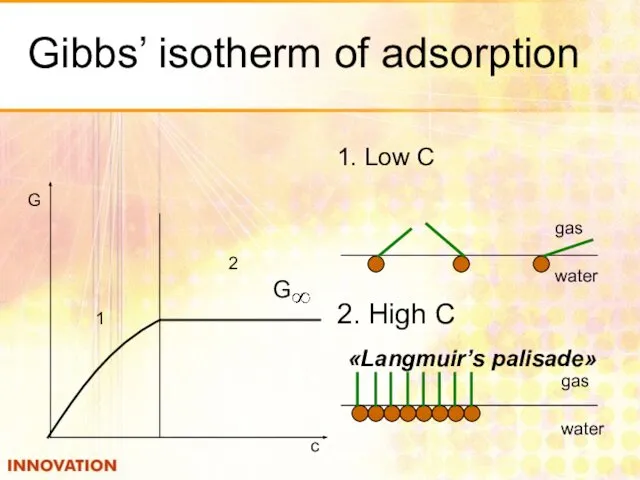
- 19. Gibbs’ isotherm of adsorption 1. Low С 1 2 G c gas water 2. High С
- 20. ADSORPTION ON THE SOLID-GAS BORDER
- 21. Adsorption by solids The adsorption value depends on: 1. The size of the adsorbent surface if
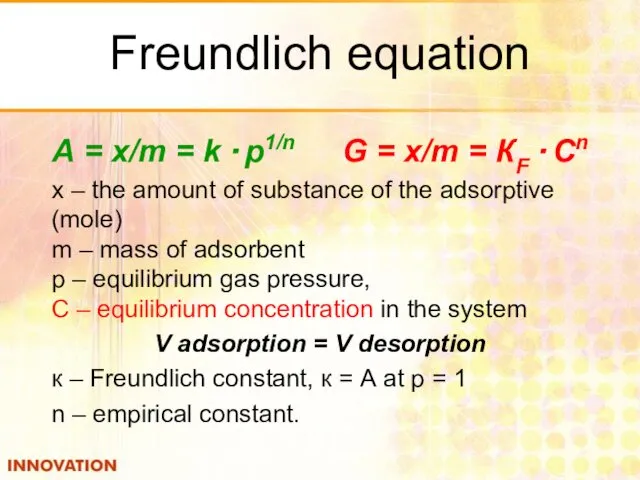
- 22. Freundlich equation А = x/m = k · p1/n G = x/m = КF · Cn
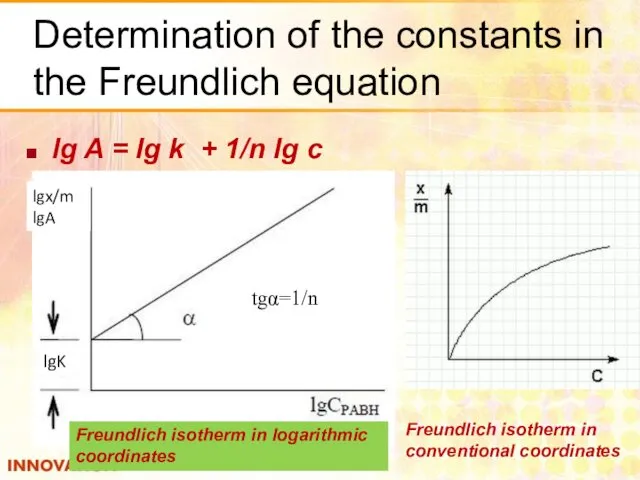
- 23. Determination of the constants in the Freundlich equation lg A = lg k + 1/n lg
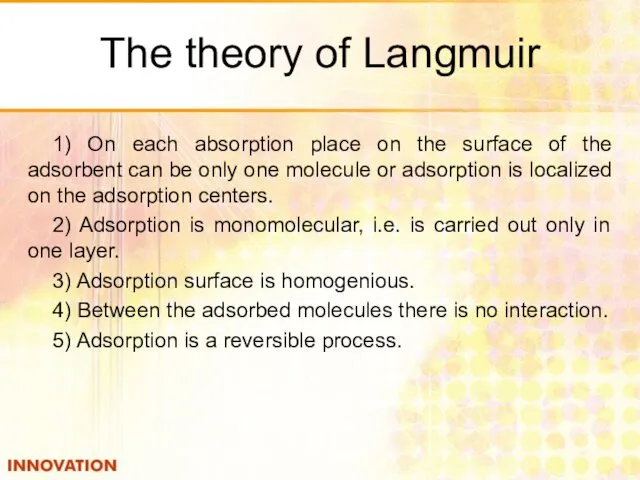
- 24. The theory of Langmuir 1) On each absorption place on the surface of the adsorbent can
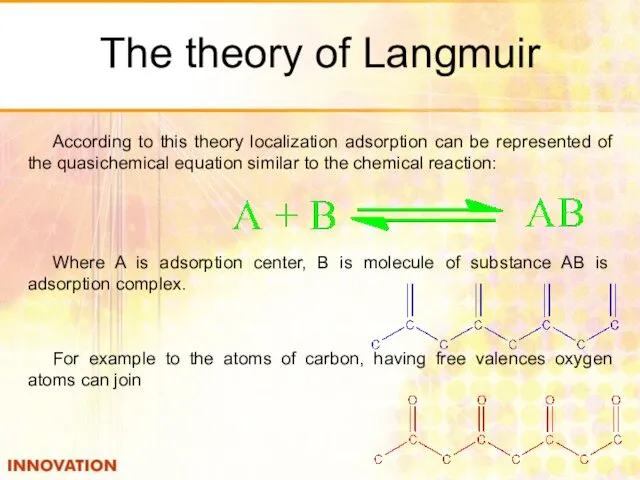
- 25. The theory of Langmuir According to this theory localization adsorption can be represented of the quasichemical
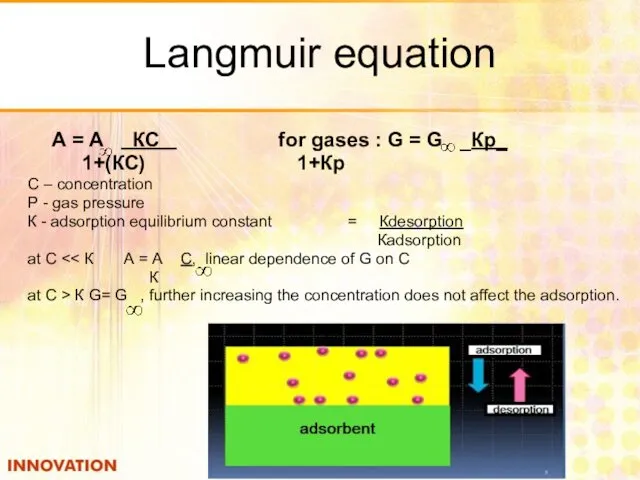
- 26. Langmuir equation А = А КС for gases : G = G _Кр_ 1+(КС) 1+Кр С
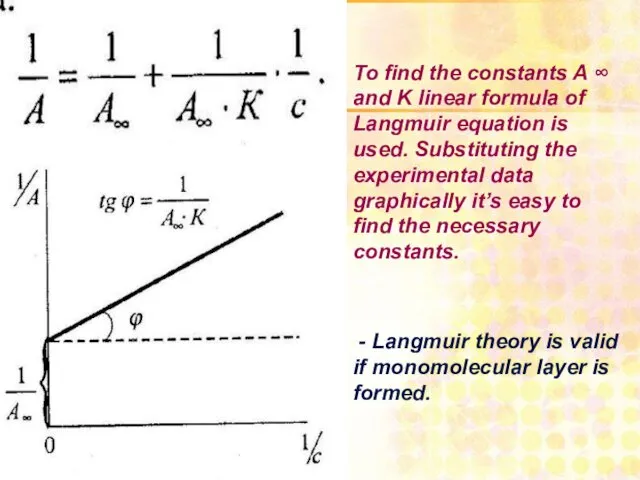
- 27. To find the constants A ∞ and K linear formula of Langmuir equation is used. Substituting
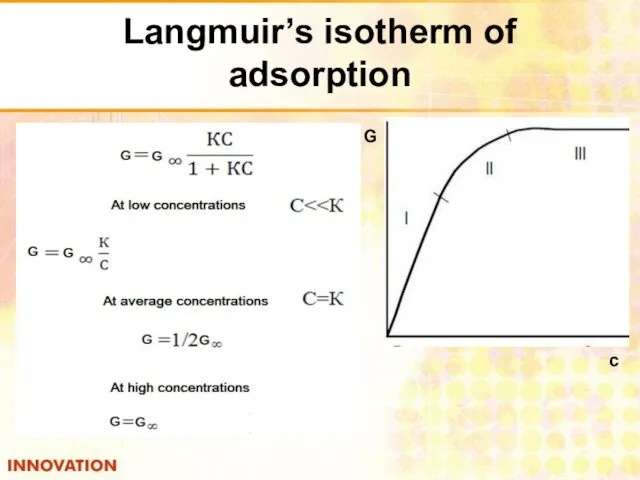
- 28. Langmuir’s isotherm of adsorption с G
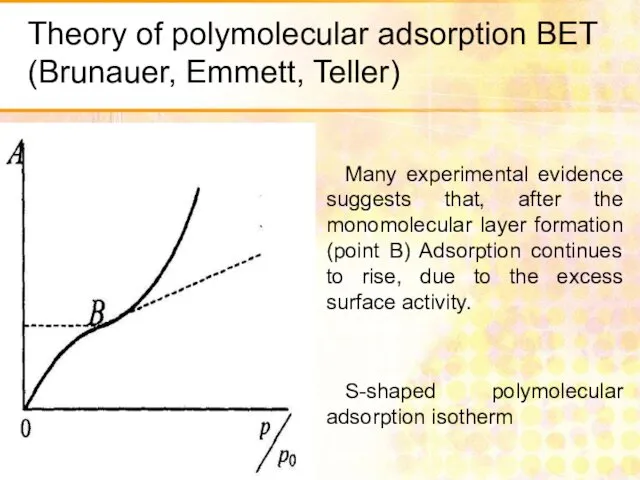
- 29. Theory of polymolecular adsorption BET (Brunauer, Emmett, Teller) Many experimental evidence suggests that, after the monomolecular
- 30. ADSORPTION ON THE BORDER OF SOLID – SOLUTION In the study of adsorption from solutions on
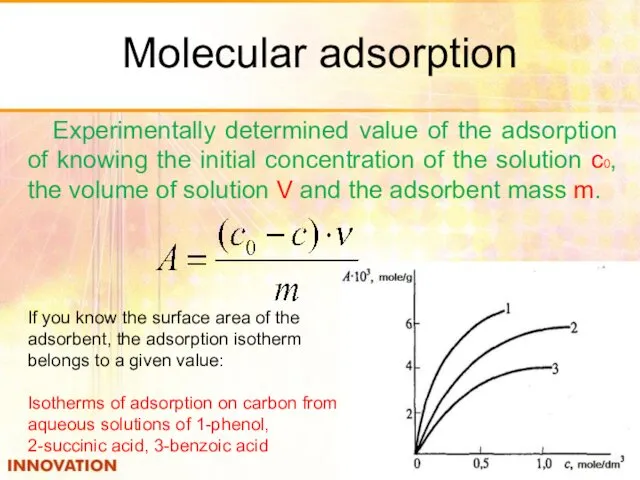
- 31. Molecular adsorption Experimentally determined value of the adsorption of knowing the initial concentration of the solution
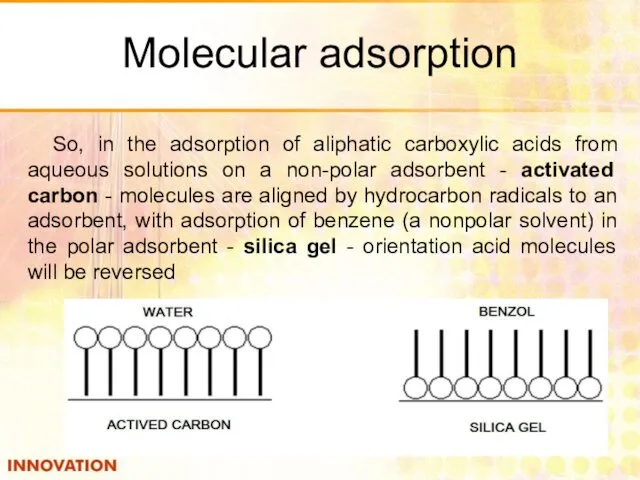
- 32. Molecular adsorption So, in the adsorption of aliphatic carboxylic acids from aqueous solutions on a non-polar
- 33. Conclusion From the above that is confirmed, that: For adsorption SAS from the nonpolar or low-polar
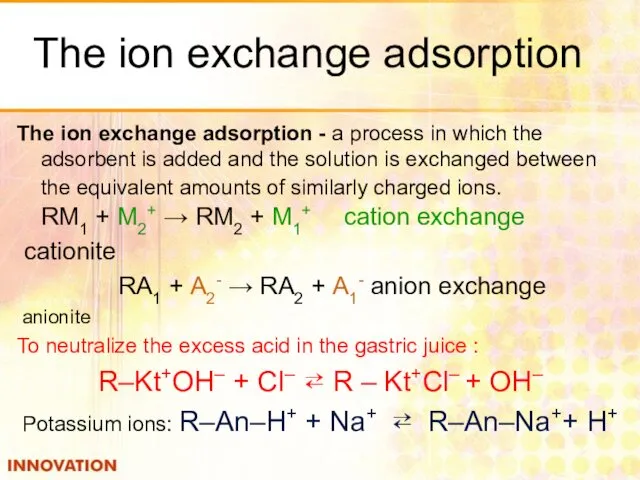
- 34. The ion exchange adsorption The ion exchange adsorption - a process in which the adsorbent is
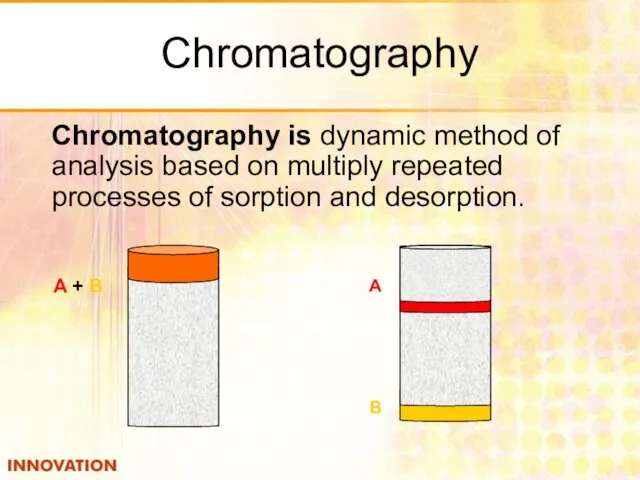
- 35. Chromatography Chromatography is dynamic method of analysis based on multiply repeated processes of sorption and desorption.
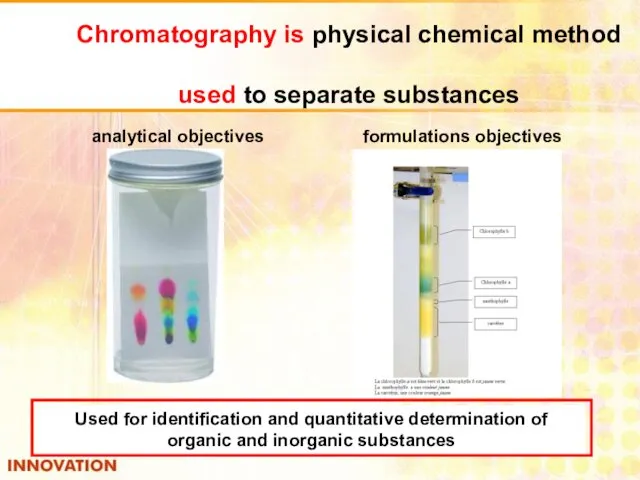
- 36. Chromatography is physical chemical method used to separate substances analytical objectives formulations objectives Used for identification
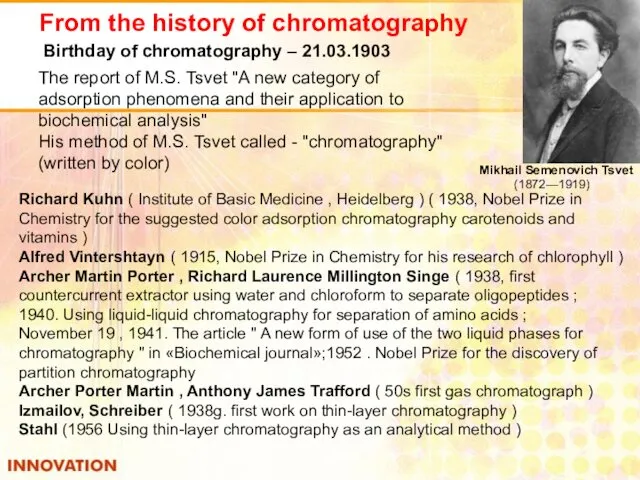
- 37. From the history of chromatography Mikhail Semenovich Tsvet (1872—1919) Birthday of chromatography – 21.03.1903 The report
- 38. «No other discovery had such a huge long lasting effect in organic chemistry as the analysis
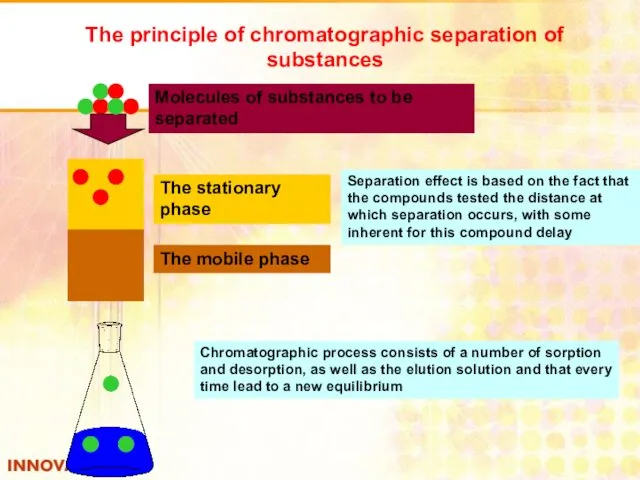
- 39. The principle of chromatographic separation of substances The stationary phase The mobile phase Molecules of substances
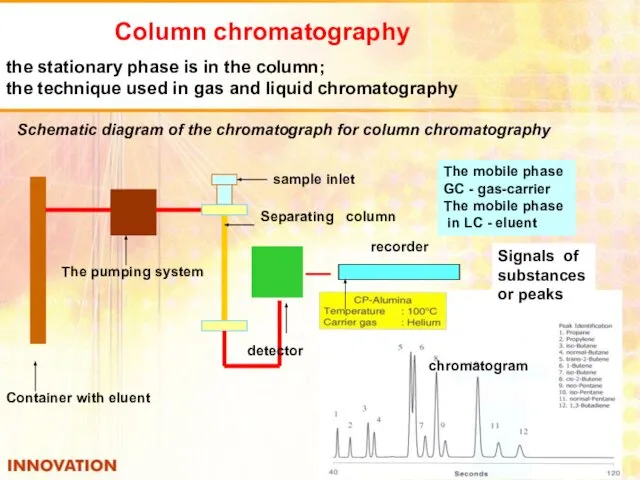
- 40. Column chromatography the stationary phase is in the column; the technique used in gas and liquid
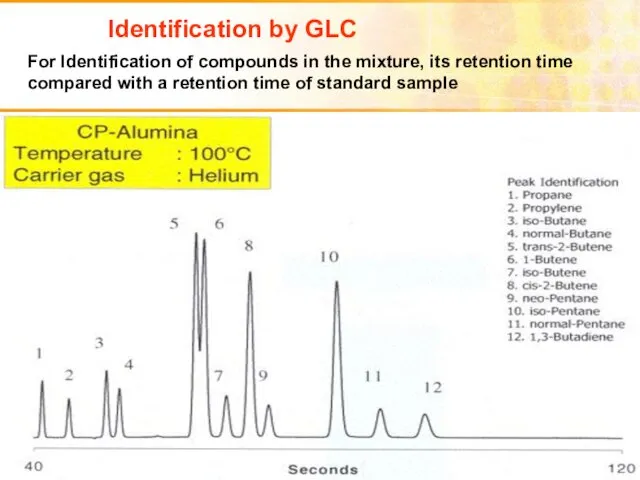
- 41. Identification by GLC For Identification of compounds in the mixture, its retention time compared with a
- 42. HPLC Agilent Technologies
- 43. HPLC Milichrom
- 44. HPLC HP
- 45. GLC “Agilent Technologies”
- 46. Enterosorption It is method of treatment of various diseases, based on the ability of enterosorbents bind
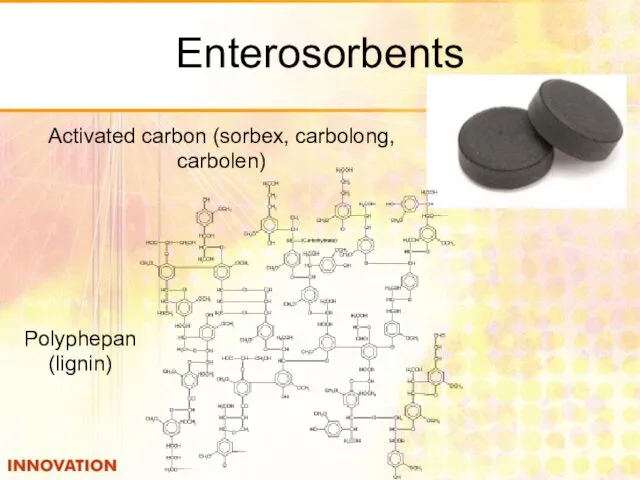
- 47. Enterosorbents Polyphepan (lignin) Activated carbon (sorbex, carbolong, carbolen)
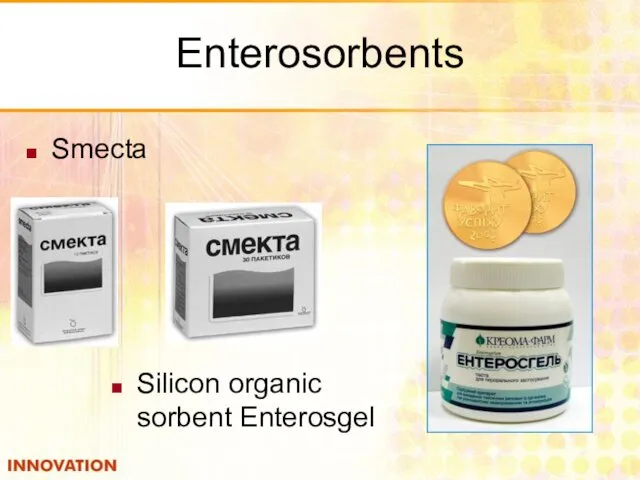
- 48. Enterosorbents Smecta Silicon organic sorbent Enterosgel
- 49. Enterosorption Enterosorption is part of efferent therapy (from the Latin word efferens means output). Also enterosorption,
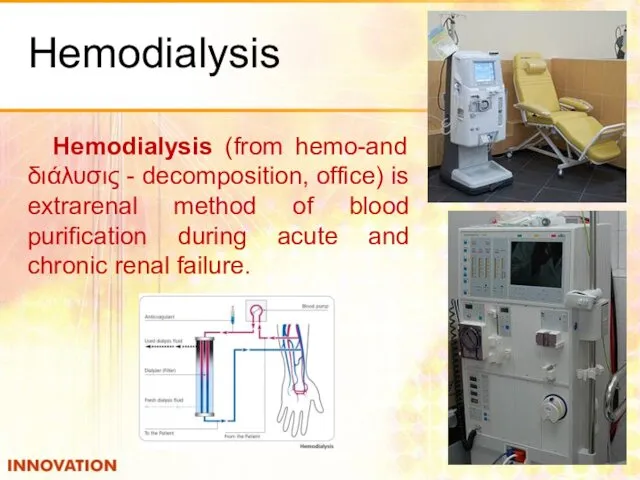
- 50. Hemodialysis Hemodialysis (from hemo-and διάλυσις - decomposition, office) is extrarenal method of blood purification during acute
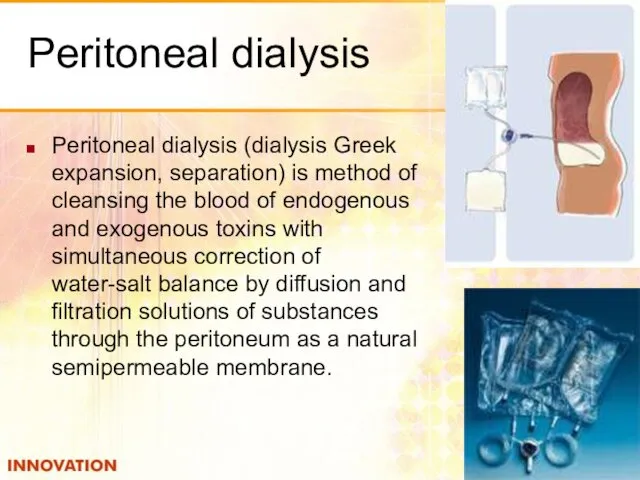
- 51. Peritoneal dialysis Peritoneal dialysis (dialysis Greek expansion, separation) is method of cleansing the blood of endogenous
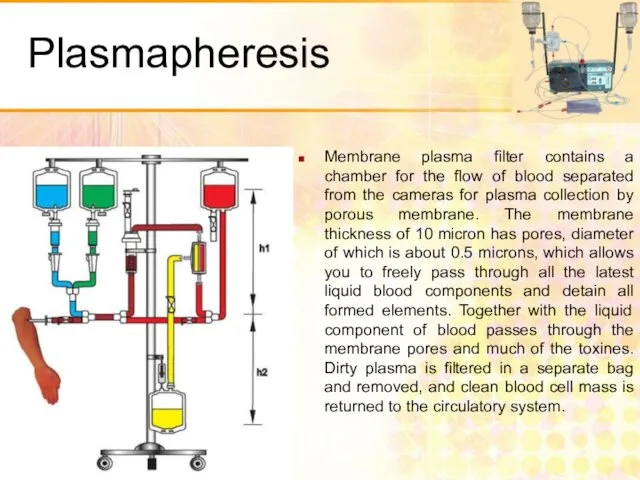
- 52. Plasmapheresis Membrane plasma filter contains a chamber for the flow of blood separated from the cameras
- 54. Скачать презентацию



![Gibbs Equation G - the amount of adsorbed substance [mole/m2] а](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/461298/slide-10.jpg)













 Фенолы. Простые эфиры
Фенолы. Простые эфиры Митохондрии и пластиды
Митохондрии и пластиды ПОЛУЧЕНИЕ СИВУШНЫХ МАСЕЛ И СПИРТА ПРИ БРОЖЕНИИ УГЛЕВОДОВ Кейс 2
ПОЛУЧЕНИЕ СИВУШНЫХ МАСЕЛ И СПИРТА ПРИ БРОЖЕНИИ УГЛЕВОДОВ Кейс 2 Химическое равновесие
Химическое равновесие Промислові способи отримання металів
Промислові способи отримання металів  Уравнения химических реакций
Уравнения химических реакций Углеводороды. Обобщение знаний
Углеводороды. Обобщение знаний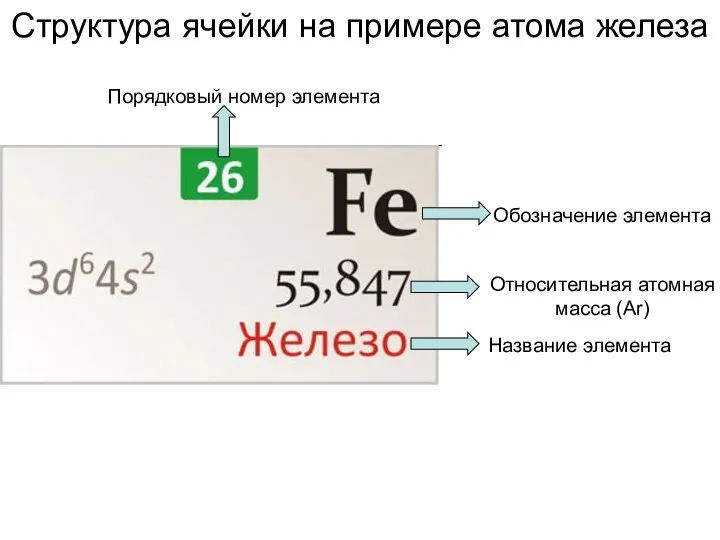 Структура ячейки на примере атома железа
Структура ячейки на примере атома железа Основы аналитической химии и физико - химических методов анализа
Основы аналитической химии и физико - химических методов анализа Общие сведения о полезных ископаемых. (Лекция 2)
Общие сведения о полезных ископаемых. (Лекция 2) ИСКУССТВЕННЫЕ ПОЛИМЕРЫ
ИСКУССТВЕННЫЕ ПОЛИМЕРЫ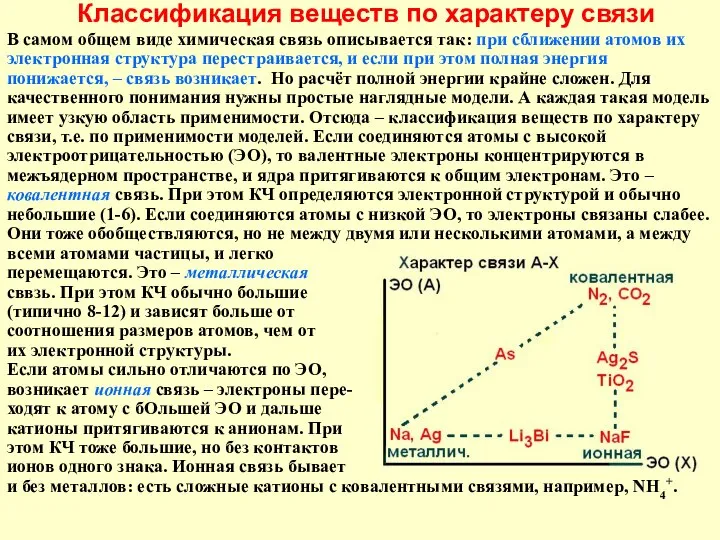 Классификация веществ по характеру связи
Классификация веществ по характеру связи Припекание взаимно растворимых твердых тел
Припекание взаимно растворимых твердых тел Строение атома фосфора. Строение оксида фосфора (V) и его физические свойства
Строение атома фосфора. Строение оксида фосфора (V) и его физические свойства Гетерофункционалды органикалық қосылыстар
Гетерофункционалды органикалық қосылыстар Дезодоранты. Выполнили: Ученицы 11-В класса МОУ «Лицей №3» Доровских Алёна Чучуменко Анастасия
Дезодоранты. Выполнили: Ученицы 11-В класса МОУ «Лицей №3» Доровских Алёна Чучуменко Анастасия Кислоты. Определение кислот
Кислоты. Определение кислот Кристаллическое строение материалов. Лекция 2
Кристаллическое строение материалов. Лекция 2 Тесты для самопроверки
Тесты для самопроверки Производство бензина
Производство бензина Использование проектного метода при изучении химии
Использование проектного метода при изучении химии Старение полимеров. Процессы, протекающие при старении полимеров
Старение полимеров. Процессы, протекающие при старении полимеров Пластмассы
Пластмассы Каустобиолиты, горючие полезные ископаемые органического происхождения
Каустобиолиты, горючие полезные ископаемые органического происхождения Теплові прояви механічної, електричної та хімічної енергії
Теплові прояви механічної, електричної та хімічної енергії Алканы CnH2n+2 - Презентация по Химии
Алканы CnH2n+2 - Презентация по Химии Химическая кинетика
Химическая кинетика Ферроцен. Свойства, получение и применение
Ферроцен. Свойства, получение и применение