Содержание
- 2. Aim Learn to prepare gaseous samples with desired concentration of a solute
- 3. Importance Preparation of calibration samples (standards) Conducting chemical reactions in gas phase Production of commercial gases
- 4. Advantages of having the skill More accurate calibration and analytical measurements Lower consumption of expensive materials
- 5. Example - quantification
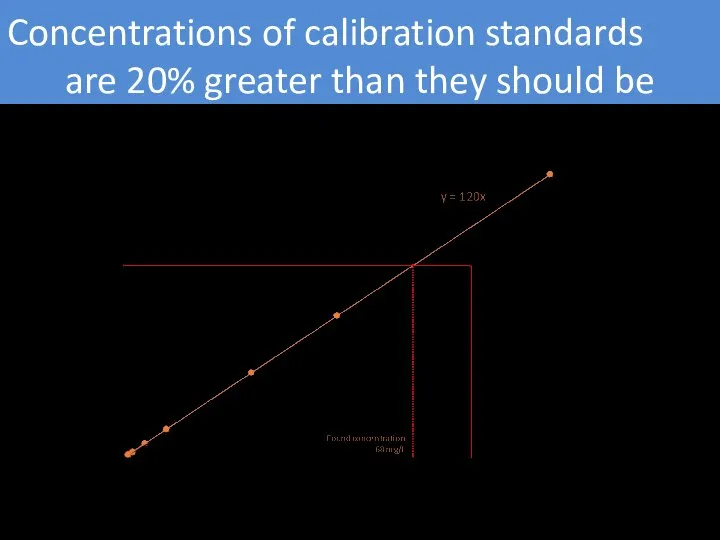
- 6. Concentrations of calibration standards are 20% greater than they should be
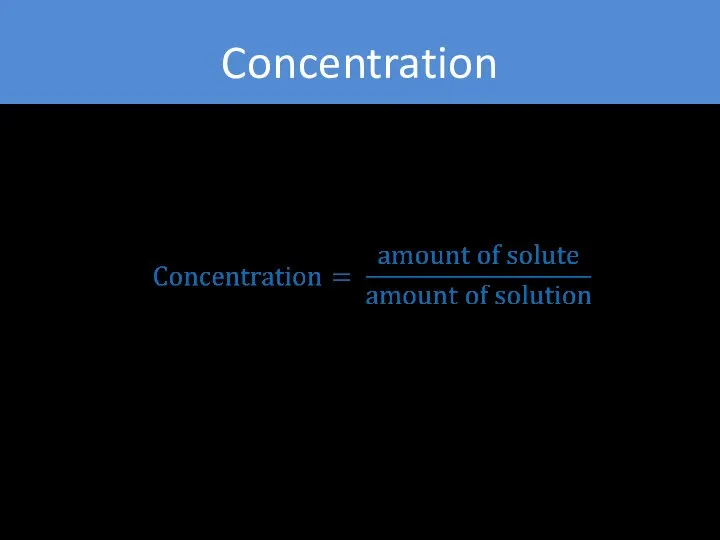
- 7. Concentration general measurement unit stating the amount of solute present in a known amount of solution
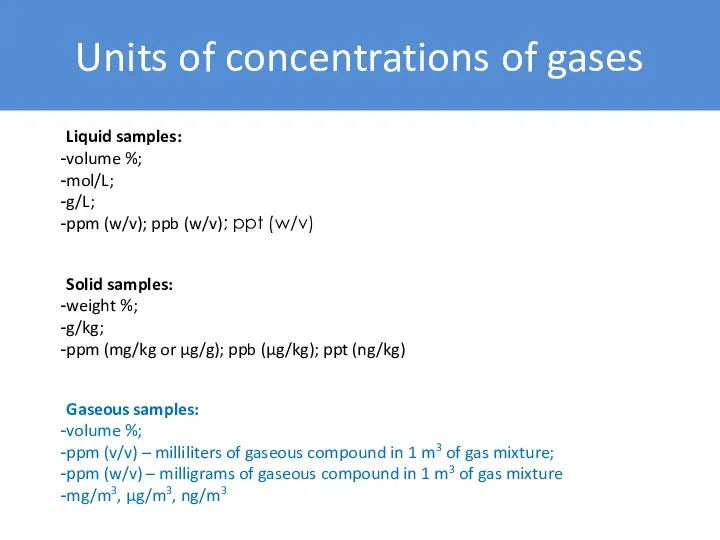
- 8. Units of concentrations of gases Liquid samples: volume %; mol/L; g/L; ppm (w/v); ppb (w/v); ppt
- 9. Types of concentrations Volume/volume – does not change with T and P Mass / volume –
- 10. Main formula for conversions p – pressure (ambient or partial), kPa V – volume, L m
- 11. Exercise Convert 50 ppm (v/v) of hydrogen sulfide in air to mg/m3 Now we need to
- 12. Solution (continued) V = 50 mL; R=8.31 L· kPa / (moL K); M (H2S) = 34
- 13. Solution (continued) Q: will the C increase if temperature is increased to 30 ⁰C?
- 14. Question What is the partial pressure of H2S at this concentr.? m = 0.0655 g; V
- 15. Quiz 1/2 1 – 37 2 – 55 3 – 25 4 - 43 5 –
- 16. Quiz 2/2 1 – 49 µg/m3 2 – 56 µg/m3 3 – 64 µg/m3 4 -
- 17. Question What equipment and glassware is used for preparing liquid solutions?
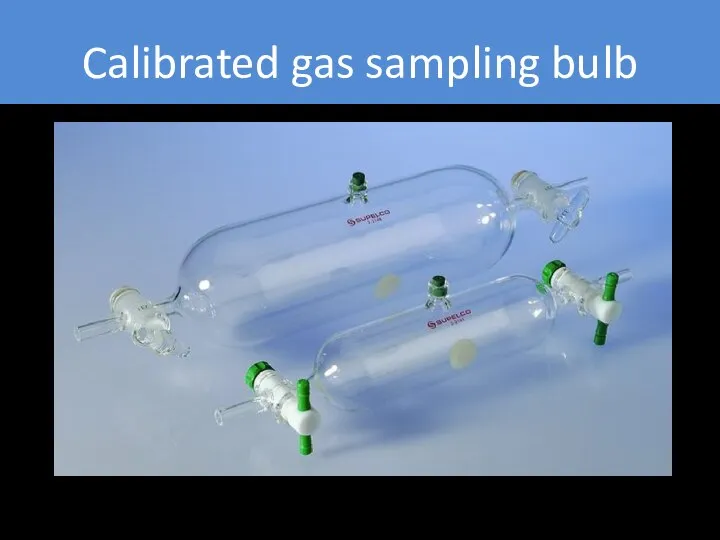
- 18. Calibrated gas sampling bulb To prepare gas standard, inject small amount (
- 19. Exercise How many nanograms of naphthalene should be injected into a 500-mL bulb filled with “zero”
- 20. Exercise (continued) What concentration should the injected solution have if the injected volume is 5.0 µL?
- 21. Exercise Solution of benzene (5.00 µL) in methanol with concentration 10 mg/mL was injected to calibrated
- 22. Task Convert this concentration to ppmV Convert this concentration to Pa
- 23. Question How many microliters of water can be introduced to a 250-mL flask containing dry air
- 24. Task 2 How many microliters of methanol can be introduced to a 250-mL flask containing air
- 25. Gas tight syringes PTFE plunger
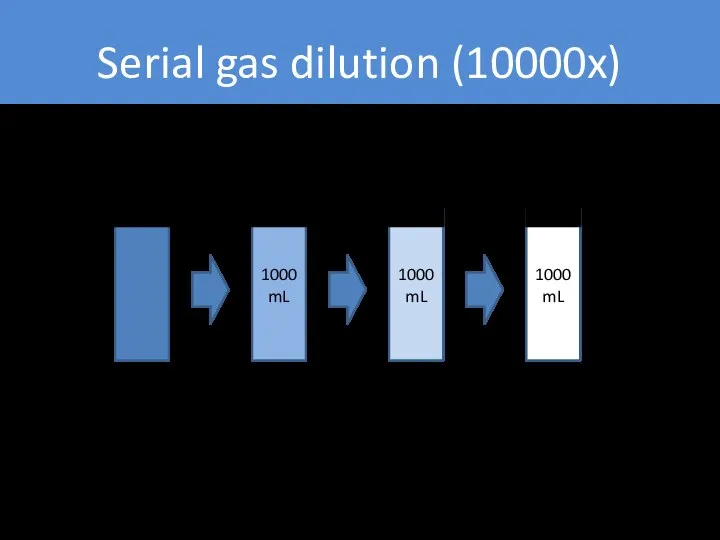
- 26. Serial gas dilution (10000x) 1000 mL 1000 mL 1000 mL Pure gas 100 µL/L (100 ppm)
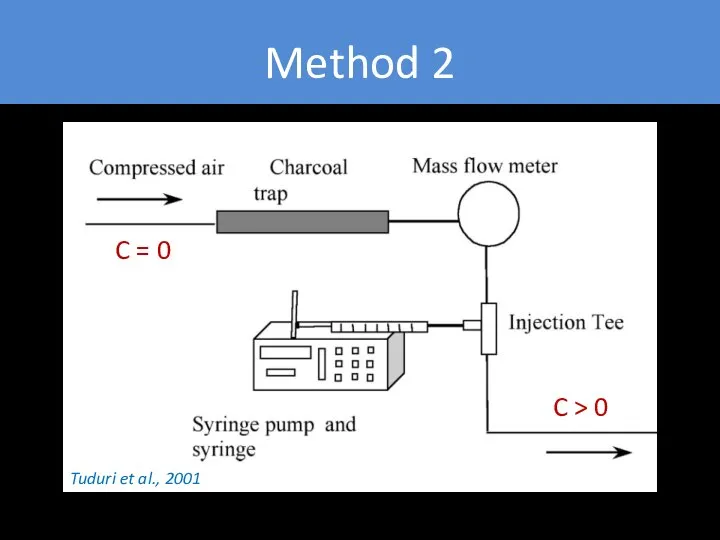
- 27. Method 2 C = 0 C > 0 Tuduri et al., 2001
- 28. New Era NE-1002X
- 29. Example “Zero” air is supplied at 100 mL/min rate Benzene solution in methanol (C = 50
- 30. Calculation
- 32. Скачать презентацию









 Синтетикалық талшықтар
Синтетикалық талшықтар Роль воды в жизнедеятельности организма. Теория растворов электролитов и неэлектролитов коллигативные свойства растворов
Роль воды в жизнедеятельности организма. Теория растворов электролитов и неэлектролитов коллигативные свойства растворов Пластмаси та полімери Євсєєнко Ганна,11-1
Пластмаси та полімери Євсєєнко Ганна,11-1  Периодический закон и периодическая система Д.И. Менделеева. Самостоятельное занятие № 2
Периодический закон и периодическая система Д.И. Менделеева. Самостоятельное занятие № 2 Йод. Йодтың ашылуы
Йод. Йодтың ашылуы Химия вопросы и ответы
Химия вопросы и ответы Исследование зависимости интенсивности люминесценции пленок оксида цинка от уровня фотовозбуждения
Исследование зависимости интенсивности люминесценции пленок оксида цинка от уровня фотовозбуждения Кислотноcть и основность органических соединений. Инфракрасная спектроскопия. Лекция № 2
Кислотноcть и основность органических соединений. Инфракрасная спектроскопия. Лекция № 2 Изучение растворимости веществ. Приготовление растворов и анализ состава растворов путем выпаривания
Изучение растворимости веществ. Приготовление растворов и анализ состава растворов путем выпаривания Гетерофункциональные органические соединения. Определение
Гетерофункциональные органические соединения. Определение Металлы
Металлы Презентация к уроку химии по теме «Дисперсные системы» для 11 класса. УМК Габриеляна
Презентация к уроку химии по теме «Дисперсные системы» для 11 класса. УМК Габриеляна Пластмаси, синтетичні каучуки Підготував учень 11 класу Петро Ферій
Пластмаси, синтетичні каучуки Підготував учень 11 класу Петро Ферій  Исследовательская работа по химии Количественное содержание витамина С в ягодах клюквы и проблема сохранности в зимний период вр
Исследовательская работа по химии Количественное содержание витамина С в ягодах клюквы и проблема сохранности в зимний период вр Вклад химиков – органиков в Победу
Вклад химиков – органиков в Победу Презентация по Химии "Атомы, молекулы, химические элементы. Формы существования элементов в природе" - скачать смотреть
Презентация по Химии "Атомы, молекулы, химические элементы. Формы существования элементов в природе" - скачать смотреть  Качественный и количественный анализ бромокриптина и его производных
Качественный и количественный анализ бромокриптина и его производных Органическая химия. Олигосахариды. Полисахариды
Органическая химия. Олигосахариды. Полисахариды Электрохимические анализаторы медицинского назначения
Электрохимические анализаторы медицинского назначения Бериллий, магний, щелочноземельные металлы
Бериллий, магний, щелочноземельные металлы Современные методы образования амидной связи с использованием ацилгалогенидов, ангидридов, активированных эфиров и их аналогов
Современные методы образования амидной связи с использованием ацилгалогенидов, ангидридов, активированных эфиров и их аналогов Выделение урана из растворов (пульп)
Выделение урана из растворов (пульп) Презентация по химии Электролитическая диссоциация
Презентация по химии Электролитическая диссоциация  Металдар дың химиялық қасиеттері. Бейметалдардың химиялық қасиеттері
Металдар дың химиялық қасиеттері. Бейметалдардың химиялық қасиеттері Охорона довкілля від забруднення під час переробки нафти та кам’яного вугілля
Охорона довкілля від забруднення під час переробки нафти та кам’яного вугілля  Железо, его физические и химические свойства
Железо, его физические и химические свойства Аэробное окисление углеводов
Аэробное окисление углеводов Калитина Тамара Михайловна учитель экологии, биологии МОУ СОШ №3 и учитель химии МОУ СОШ №2 с.Александров-Гай Саратовской области
Калитина Тамара Михайловна учитель экологии, биологии МОУ СОШ №3 и учитель химии МОУ СОШ №2 с.Александров-Гай Саратовской области